
����MnO2��CuSO4��Һ������H2O2��Һ�ֽ�Ĵ�����ijУ��ѧ��ȤС����̽������һЩ���������������Һ�Ƿ�Ҳ������H2O2��Һ�ֽ�Ĵ���������������ǵ�̽�����̡�
��1��[С���IJ���]��Al2O3������������ֽ�Ĵ�����
[С���IJ���]��FeCl3��Һ������������ֽ�Ĵ�����
��2��[ʵ����֤]��
| ʵ�鲽�� | ʵ������ | ʵ����� | |
| С����ʵ�� | �������ǵ�ľ������װ�й���������Һ���Թ��� | ľ������ȼ |
|
|
| ľ����ȼ | Al2O3�ܼӿ�H2O2��Һ�ķֽ����� | |
| С����ʵ�� | ��һ֧�Թ��м���H2O2��Һ��Ȼ�����������FeCl3��Һ���Ѵ����ǵ�ľ�������Թܡ� |
| FeCl3��Һ�ӿ�H2O2��Һ�ķֽ����� |
��3��[����]����Al2O3��FeCl3��Һ����H2O2��Һ�ֽ�Ĵ�����
��4��[��˼1]���е�ͬѧ��ΪС����ʵ�飬������ȫ֤��Al2O3��H2O2��Һ�ֽ������˴����á���Ӧ������̽��Al2O3 ��
[��˼2]����֪FeCl3��ˮ�пɽ����Fe3+ ��Cl-��ͬѧ��������²��룺
��ͬѧ�IJ����ǣ��������ֽ�H2O2����FeCl3��Һ�е�H2O��
��ͬѧ�IJ����ǣ��������ֽ�H2O2����FeCl3��Һ�е�Fe3+��
��ͬѧ�IJ����ǣ��������ֽ�H2O2����FeCl3��Һ�е�Cl-��
����Ϊ����ܵ��� ͬѧ�IJ��룬 ������
��5��ͬѧ�Ƕ����µ��������룬��ʵ�������̽����������ϸ�����������
| ʵ����� | ʵ������ | ���� |
| ��ʢ��5ml5%��H2O2��Һ���Թ��м������� ��HCl�����Ѵ����ǵ�ľ�������Թܡ� | ���������� |
|
| ��ʢ��5ml5%��H2O2��Һ���Թ��м������� �� �����Ѵ����ǵ�ľ�������Թܡ� |
|
|

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鲽�� | ʵ������ | ʵ����� | |
| С����ʵ�� | �������ǵ�ľ������װ�й���������Һ���Թ��� | ľ������ȼ | �����¹�������ֽ����ʺ��� �����¹�������ֽ����ʺ��� |
װ�й���������Һ���Թ��м�������Al2O3 װ�й���������Һ���Թ��м�������Al2O3 Ȼ�����ǵ�ľ�������Թ��� Ȼ�����ǵ�ľ�������Թ��� |
ľ����ȼ | Al2O3�ܼӿ�H2O2��Һ�ķֽ����� | |
| С����ʵ�� | ��һ֧�Թ��м���H2O2��Һ��Ȼ�����������FeCl3��Һ���Ѵ����ǵ�ľ�������Թܣ� | Ѹ�ٲ����������� Ѹ�ٲ����������� �����ǵ�ľ����ȼ �����ǵ�ľ����ȼ |
FeCl3��Һ�ӿ�H2O2��Һ�ķֽ����� |
| ʵ����� | ʵ������ | ���� |
| ��ʢ��5ml5%��H2O2��Һ���Թ��м������� ��HCl�����Ѵ����ǵ�ľ�������Թܣ� |
���������� | Cl-���ܴ��ֽ�H2O2 Cl-���ܴ��ֽ�H2O2 |
| ��ʢ��5ml5%��H2O2��Һ���Թ��м������� �� Fe��NO3��3��Һ Fe��NO3��3��Һ �����Ѵ����ǵ�ľ�������Թܣ� |
�Թ����д������ݲ����������ǵ�ľ����ȼ �Թ����д������ݲ����������ǵ�ľ����ȼ |
������õ���Fe3+ ������õ���Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
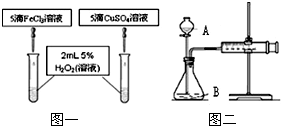
 ����Уʵ����ʵʩ��������2011���п���ѧʵ���������ո������Ļ��ʵ�鿼����H2O2�ֽ�������Ϊ�β�����MnO2��������ͬѧ��Ϊ֮չ����̽����
����Уʵ����ʵʩ��������2011���п���ѧʵ���������ո������Ļ��ʵ�鿼����H2O2�ֽ�������Ϊ�β�����MnO2��������ͬѧ��Ϊ֮չ����̽����| ���������� | O2 ƽ������ | ��������ʱ�� | �ɱ� | ��Ӧ��� |
| MnO21.0g | 919mL | 10��03�� | 0.11Ԫ | �ֽ��ȿ���� |
| 10%FeCl3��Һ5�� | 985mL | 4��50�� | 0.02Ԫ | ���Ȳ���O2 |
| 15%CuSO4��Һ5�� | 955mL | 4��57�� | 0.03Ԫ | ���Ȳ���O2 |
| ||
| ||
| Ũ�� ʱ�䣨min�� ���� |
30% H2O2 | 15% H2O2 | 5% H2O2 |
| ����a g MnO2 | 0.2 | 0.8 | 2.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�鲽�� | ʵ������ | ʵ����� | |
| С����ʵ�� | �������ǵ�ľ������װ�й���������Һ���Թ��� | ľ������ȼ | ______ |
| ______ ______ | ľ����ȼ | Al2O3�ܼӿ�H2O2��Һ�ķֽ����� | |
| С����ʵ�� | ��һ֧�Թ��м���H2O2��Һ��Ȼ�����������FeCl3��Һ���Ѵ����ǵ�ľ�������Թܣ� | ______ ______ | FeCl3��Һ�ӿ�H2O2��Һ�ķֽ����� |
| ������ ʵ����� | ʵ������ | ���� |
| ��ʢ��5ml5%��H2O2��Һ���Թ��м������� ��HCl�����Ѵ����ǵ�ľ�������Թܣ� | ���������� | ______ |
| ��ʢ��5ml5%��H2O2��Һ���Թ��м������� ��______�����Ѵ����ǵ�ľ�������Թܣ� | ______ | ______ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��ͷ��������ѧ���꼶���ϣ�����ѧ�¿���ѧ�Ծ��������棩 ���ͣ������
| ʵ�鲽�� | ʵ������ | ʵ����� | |
| С����ʵ�� | �������ǵ�ľ������װ�й���������Һ���Թ��� | ľ������ȼ | |
| | ľ����ȼ | Al2O3�ܼӿ�H2O2��Һ�ķֽ����� | |
| С����ʵ�� | ��һ֧�Թ��м���H2O2��Һ��Ȼ�����������FeCl3��Һ���Ѵ����ǵ�ľ�������Թܣ� | | FeCl3��Һ�ӿ�H2O2��Һ�ķֽ����� |
| ʵ����� | ʵ������ | ���� |
| ��ʢ��5ml5%��H2O2��Һ���Թ��м������� ��HCl�����Ѵ����ǵ�ľ�������Թܣ� | ���������� | |
| ��ʢ��5ml5%��H2O2��Һ���Թ��м������� �� �����Ѵ����ǵ�ľ�������Թܣ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com