
| 14”Į2 |
| 132 |
| 14”Į2 |
| 14”Į2+1”Į4+16”Į3 |
| 14”Į2 |
| 132 |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
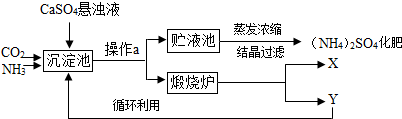
| ||
| Öø±ź ĻīÄæ |
ÓŵČĘ· | ŗĻøńĘ· |
| µŖ£ØN£©ŗ¬Įæ | ”Ż21.0% | ”Ż20.5% |
²éæ““š°øŗĶ½āĪö>>
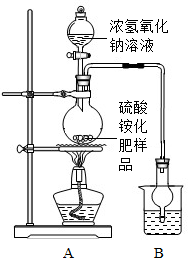
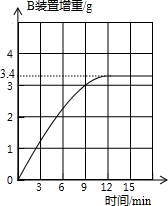
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| NµÄĻą¶ŌŌ×ÓÖŹĮæ”Į2 |
| (NH2)2SO4µÄĻą¶Ō·Ö×ÓÖŹĮæ |
| 14”Į2 |
| 132 |
| 7.08g |
| 7.5g |
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā

| ŗÓ¶Ī±ąŗÅ | Ė®ÖŹĄą±š | Ö÷ŅŖĪŪČ¾Öø±ź | ÖŹĮæדæö |
| ¼× | ¢ņ | Į¼ŗĆ | |
| ŅŅ | ¢ō | ¹Æ | Ņ»°ć |
| ±ū | ĮÓ¢õ | ×ÜĮ×”¢µŖµČ | ½Ļ²ī |
| ÓīÖęÅĘ»Æ·Ź--¹ś¼ŹŹ×““ Ö÷ŅŖ³É·Ö£ŗÄņĖŲ[CO£ØNH2£©2] ŗ¬µŖĮæ¾ÓČ«Ēņ»Æ·ŹÖ®Ź×£ŗ48% ¾»ŗ¬Įæ£ŗ50kg/“ü ”°ÓīÖę”±»Æ·ŹÓŠĻŽŌšČĪ¹«Ė¾³öĘ· |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĮņĖįļ§µÄ»ÆѧŹ½ĪŖ£ØNH4£©2SO4£¬
£Ø1£©ĮņĖįļ§µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ____________£»
£Ø2£©N”¢H”¢S”¢OµÄŌ×ÓøöŹż±Č_______________£»
£Ø3£©N”¢H”¢S”¢OµÄÖŹĮæ±Č____________£»
£Ø4£©µŖŌŖĖŲµÄÖŹĮæ·ÖŹż____________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com