
£Ø5·Ö£©¹žÄĻŠĀ³ĒŌŚµŲĢś½ØÉčÖŠŠčŅŖ“óĮæµÄøÖĢś£¬ĻÖÓŠ³ąĢśæóѳʷ£ØĖłŗ¬ŌÓÖŹ²»ČÜÓŚĖ®Ņ²²»ÓėĖį·“Ó¦£©£¬ŹµŃ銔×éĶ¬Ń§ĪŖ²ā¶ØĘä“æ¶Č£¬
½ųŠŠĮĖČż“ĪŹµŃ飬ŹµŃ鏿¾ŻČēĻĀ±ķĖłŹ¾”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
| | µŚ1“Ī | µŚ2“Ī | µŚ3“Ī |
| ѳʷ֏Įæ£Øg£© | 25 | 30 | 25 |
| ŃĪĖįÖŹĮæ£Øg£© | 200 | 100 | 100 |
| ĖłµĆČÜŅŗÖŹĮæ£Øg£© | 216 | 116 | 116 |
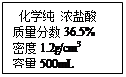
£Ø1£©Fe2O3+6HCl===2FeCl3+3H2O £Ø2£©=
£Ø3£©20% £Ø4£©200 £Ø5£©404t
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©³ąĢśæóµÄÖ÷ŅŖ³É·ÖŹĒŃõ»ÆĢś£¬ÓėŃĪĖį·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗFe2O3 + 6HCl = 2FeCl3 + 3H2O
£Ø2£© øł¾ŻČż“ĪŹµŃ鏿¾Ż·ÖĪöµŚČż“Ī³ąĢśæóѳʷ֊µÄŃõ»ÆĢśĒ”ŗĆÓė100gŃĪĖį·“Ó¦ĶźČ«£¬øł¾ŻĖłµĆČÜŅŗµÄÖŹĮ棬æÉÖŖ25g³ąĢśæóѳʷ֊µÄŃõ»ÆĢśµÄÖŹĮæĪŖ16g£¬ĖłŅŌøł¾Ż·½³ĢŹ½£ŗFe2O3+6HCl==2FeCl3+3H2OÖŠFe2O3ÓėHClµÄÖŹĮæ¹ŲĻµ160:219£¬ĖłŅŌµŚ2“Ī²Ī¼Ó·“Ó¦µÄŃĪĖįÖŠČÜÖŹÖŹĮæ(X)µÄ±ČĄżŹ½£ŗ160:219=16g£ŗx
£Ø3£© øł¾ŻÉĻĆę·ÖĪö£¬µŚČż“Ī³ąĢśæóѳʷ֊µÄŃõ»ÆĢśĒ”ŗĆÓė100gŃĪĖį·“Ó¦ĶźČ«£¬æÉøł¾Ż·½³ĢŹ½£ŗFe2O3+6HCl==2FeCl3+3H2O£¬ĻČĒó³öČÜÖŹµÄÖŹĮæ
½ā£ŗÉčFeCl3µÄÖŹĮæĪŖx
Fe2O3+6HCl==2FeCl3+3H2O
325
16g x
160£ŗ325=16g£ŗx x=32£®5g
ČÜŅŗµÄÖŹĮæ=116g+46£®5g=162£®5g
ĖłŅŌĖłµĆµÄČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż=32£®5g/162£®5g”Į100%=20%
£Ø4£©ĶعżµŚ¶žĪŹµÄ·ÖĪö£¬100gŃĪĖįÖŠĖłŗ¬ČÜÖŹĪŖ21£®9g, Čż“ĪŹµŃéÖŠĖłŠčµÄŃĪĖį400g£¬¼“ČÜÖŹÖŹĮæ=21£®9g”Į4=87£®6g£¬ĖłæÉÉčĖłŠčÅØŃĪĖįµÄĢå»żĪŖv,æÉĮŠŹ½ĪŖ£ŗV”Į1£®2g/mL”Į36£®5%=87£®6g,V=200g
£Ø5£©ŅņĪŖ25g³ąĢśæóѳʷ֊µÄŃõ»ÆĢśµÄÖŹĮæĪŖ16g £¬ĖłŅŌ500tøĆæóŹÆŗ¬Ńõ»ÆĢśµÄÖŹĮæ=320t,¼“ŌÓÖŹÖŹĮæ=500t-320t=180t£¬¶ųĢśµÄÖŹĮæ=320t”Į112/160”Į100%=224t£¬ĖłŅŌŗ¬ŌÓÖŹµÄĢśµÄÖŹĮæĪŖ=180t+224t=404t
æ¼µć£ŗŹµŃ鏿¾Ż·ÖĪöŗĶ“¦Ąķ£¬øł¾Ż»Æѧ·½³ĢŹ½¼ĘĖć


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Š”ĒæČ„ÉĢµźĀņ»ŲŅ»°ü“æ¼ī£¬°ü×°ĖµĆ÷ČēÓŅĶ¼£®ĪŖ²ā¶ØøĆ“æ¼īÖŠĢ¼ĖįÄĘŗ¬ĮæŹĒ·ń·ūŗĻ±źĒ©ŅŖĒó£¬Ėū³ĘČ”ĮĖ“æ¼īѳʷ11g£¬Č«²æČܽāŌŚ50gĖ®ÖŠ£¬µ±¼ÓČėĻ”ŃĪĖį64.4gŹ±£¬Ē”ŗĆĶźČ«·“Ó¦£¬ĖłµĆČÜŅŗµÄÖŹĮæĪŖ121g£®ŹŌĒó£ŗ
£Ø1£©·“Ó¦·Å³öµÄCO2µÄÖŹĮæĪŖ g”£
£Ø2£©“æ¼īѳʷ֊Ģ¼ĖįÄʵÄÖŹĮ攣
£Ø3£©øł¾ŻÕāŅ»“Ī¼ģ²ā£¬¼ĘĖć²¢ÅŠ¶Ļѳʷ֊Ģ¼ĖįÄʵÄŗ¬ĮæŹĒ·ń·ūŗĻ±źĒ©ŅŖĒó”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
Ļņ50g Na2CO3ČÜŅŗÖŠÖšµĪ¼ÓČėŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄCaCl2ČÜŅŗ”£ŹµŃé¹ż³ĢÖŠ£¬Éś³É³ĮµķÖŹĮæÓė¼ÓČėCaCl2ČÜŅŗµÄÖŹĮæ¹ŲĻµČēÓŅĶ¼ĖłŹ¾£¬ŹŌ¼ĘĖć£ŗ
£Ø1£©Ē”ŗĆĶźČ«·“Ó¦Ź±£¬Éś³É³ĮµķµÄÖŹĮæĪŖ______________g”£
£Ø2£©¼ĘĖćĒ”ŗĆĶźČ«·“Ó¦Ź±£¬ĖłµĆČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
100æĖijÅØ¶ČµÄĮņĖįĒ”ŗĆÓė13æĖµÄŠæĶźČ«Ęš·“Ó¦£¬Ēė¼ĘĖć£ŗ
£Ø1£©Éś³ÉĒāĘųµÄÖŹĮæ£Ø½į¹ū¾«Č·µ½0.1g£©£®
£Ø2£©½«£Ø1£©µÄ½į¹ū±źŌŚĶ¼ÖŠ£»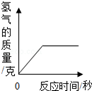
£Ø3£©·“Ó¦ŗóĖłµĆČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż£ØŠ“³ö¼ĘĖć¹ż³Ģ£¬½į¹ū¾«Č·µ½0.1%£©£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
Ēė»Ų“šĻĀĮŠÓė½šŹōÓŠ¹ŲµÄĪŹĢā£ŗ
£Ø1£©ÉśĢśĘ¬Óė“æĢśĘ¬Ļą»„æĢ»Ź±£¬“æĢśĘ¬±ķĆęæÉĮōĻĀ»®ŗŪ£¬ĖµĆ÷”” ””µÄÓ²¶Č“ó£»
£Ø2£©6.5gÓė×ćĮæµÄĻ”ĮņĖį·“Ó¦£Ø»Æѧ·½³ĢŹ½ĪŖZn+H2SO4ØTZnSO4+H2”ü£©£¬ĄķĀŪÉĻæÉÖʵĆĒāĘųµÄÖŹĮæ
ĪŖ”” ””g£®
£Ø3£©Ź¹ÓĆĻĀĮŠø÷×éŹŌ¼Į£¬ÄÜŃéÖ¤Fe”¢Cu”¢Ag»ī¶ÆŠŌĖ³ŠņµÄŹĒ”” ””
A£®Ag”¢FeSO4ČÜŅŗ”¢CuSO4ČÜŅŗ
B£®Fe”¢Cu”¢AgNO3ČÜŅŗ
C£®Fe”¢Ag”¢CuSO4ČÜŅŗ£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
£Ø7·Ö£©ŠĖȤŠ”×éµÄĶ¬Ń§ĪŖ²ā¶ØijŅ»ĢśĶŗĻ½šÖŠŗ¬ĢśµÄÖŹĮæ·ÖŹż£¬½«6gøĆŗĻ½š·Ūĩѳʷ£¬¼ÓČėČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄĮņĖįĶČÜŅŗ160gÖŠ£¬¶žÕßĒ”ŗĆĶźČ«·“Ó¦”£Ķ¬Ź±ĪŖĮĖ³ä·ÖĄūÓĆ׏Ō“£¬»¹¶Ō·“Ó¦ŗóµÄĪļÖŹ½ųŠŠ»ŲŹÕ“¦Ąķ”£Ēė½įŗĻĻĀĮŠĶ¼Ź¾¼ĘĖć£ŗ
£Ø1£©øĆŗĻ½šŃłĘ·ÖŠŗ¬ĢśµÄÖŹĮæ·ÖŹż£»£Ø¼ĘĖć½į¹ū¾«Č·ÖĮ0.1%£©
£Ø2£©ĖłµÄ¹ĢĢåĶµÄÖŹĮæaĪŖ¶ąÉŁg£æ
£Ø3£©Ļņ²»±„ŗĶĀĖŅŗÖŠ¼ÓČė¶ąÉŁgĖ®£¬ÄܵƵ½5%µÄĮņĖįŃĒĢśČÜŅŗ£¬ÓĆÓŚ»Ø»ÜµÄÓŖŃųŅŗ”£
£Ø£Ø2£©£Ø3£©ÖŠ¼ĘĖć½į¹ū¾«Č·ÖĮ0.1g£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
(7·Ö)Ė®ŹĒÉśĆüÖ®Ō“£¬°®»¤”¢ÕäĻ§Ė®×ŹŌ“ŹĒĆæøö¹«ĆńµÄŌšČĪ”£
£Ø1£©×ŌČ»½ēÖŠµÄĖ®²»ŹĒ“æĖ®£¬æÉĄūÓĆ³Įµķ”¢ ”¢Īüø½ŗĶÕōĮóµČ·½·ØæÉŅŌ¾»»ÆĖ®”£
£Ø2£©Ė®³£ÓĆĄ“ÅäÖĘø÷ÖÖČÜŅŗ£¬ĻĀĮŠĪļÖŹČÜÓŚĖ®ŗóČÜŅŗĪĀ¶ČĆ÷ĻŌÉżøߵďĒ ”£
| A£®ĒāŃõ»ÆÄĘ | B£®ĻõĖį¼Ų | C£®ĻõĖįļ§ | D£®ÅØĮņĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
ij¹«Ė¾Éś²ś³öµÄ“æ¼ī²śĘ·ÖŠ¾¼ģ²āÖ»ŗ¬ÓŠĀČ»ÆÄĘŌÓÖŹ”£ĪŖ²ā¶Ø²śĘ·ÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬20”ꏱ£¬³ĘČ”øĆ²śĘ·ŃłĘ·26£®5g£¬¼ÓČėµ½Ź¢ÓŠŅ»¶ØÖŹĮæĻ”ŃĪĖįµÄÉÕ±ÖŠ£¬Ģ¼ĖįÄĘÓėĻ”ŃĪĖįĒ”ŗĆĶźČ«·“Ó¦£¬ĘųĢåĶźČ«ŅŻ³ö£¬µĆµ½²»±„ŗĶNaClČÜŅŗ”£·“Ó¦¹ż³ĢÓĆ¾«ĆÜŅĒĘ÷²āµĆÉÕ±ÄŚ»ģŗĻĪļµÄÖŹĮæ£Øm£©Óė·“Ó¦Ź±¼ä£Øt£©¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£
Ēó£ŗ£Ø1£©Éś³ÉCO2µÄÖŹĮ攣
£Ø2£©·“Ó¦ŗóĖłµĆČÜŅŗÖŠNaClµÄÖŹĮæ·ÖŹż”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
Ēėøł¾Ż»Æѧ·½³ĢŹ½¼ĘĖć£ŗĶźČ«·Ö½ā340gČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄ¹żŃõ»ÆĒāČÜŅŗÖŠµÄ¹żŃõ»ÆĒā£ØH2O2£©£¬×ī¶ąæÉÉś³ÉŃõĘųÖŹĮæŹĒ
| A£®16g | B£®160g | C£®32g | D£®1.6g |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com