
�����������ơ���Ȫ�ڶ࣬���������� Ȫ���С�
Ȫ���С�
(1)��Ȫˮ��pH��7.5��8.9֮�䣬����Ȫˮ��________������ԡ��������ԡ������ԡ�����������Ȫˮ��Ӳˮ������ˮ�ļ����Ǽ���______�����۲측������ĭ�Ķ��١�
(2)Ϊ�ˡ������ж������������ͳ����ȼ�϶���Ϊ��Ȼ����
��ȼ�ϸ�Ϊ��Ȼ���ĺô��� ��
����Ȼ��ȼ�յĻ�ѧ����ʽ ��
(3)���꽭��ɳƺ������ˮ����ˮԴ������ˮ��������������ͼ��ʾ��
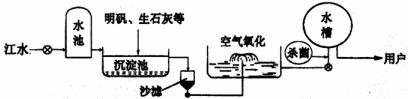 |
������ʯ�ҿɽ���ˮ��Ӳ�ȡ���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ�� ��
����ˮ�����У�������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������
Cl2+ H2O =HCIO+ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ơ���Ȫ�ڶ࣬���������� Ȫ���С�
Ȫ���С�
(1)��Ȫˮ��pH��7.5��8.9֮�䣬����Ȫˮ��________������ԡ��������ԡ������ԡ�����������Ȫˮ��Ӳˮ������ˮ�ļ����Ǽ���______�����۲측������ĭ�Ķ��١�
(2)Ϊ�ˡ������ж������������ͳ����ȼ�϶���Ϊ��Ȼ����
��ȼ�ϸ�Ϊ��Ȼ���ĺô��� ��
����Ȼ��ȼ�յĻ�ѧ����ʽ ��
(3)���꽭��ɳƺ������ˮ����ˮԴ������ˮ��������������ͼ��ʾ��
 |
������ʯ�ҿɽ���ˮ��Ӳ�ȡ���ʯ����ˮ��Ӧ�Ļ�ѧ����ʽΪ�� ��
����ˮ�����У�������������ɱ���������뽫������ˮ��Ӧ�Ļ�ѧ����ʽ����������
Cl2+ H2O =HCIO+ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com