
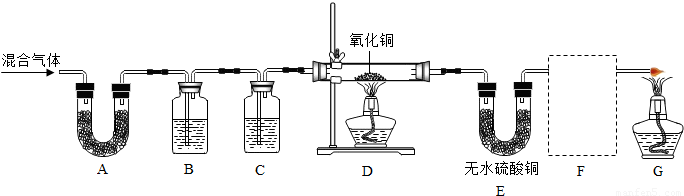
| ŹµŃé²½Öč | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ | |
| ¢Ł£Ø19£© | ŌŚ¼ā×ģµ¼¹Ü“¦µćČ¼ĘųĢ壬______£® | ______ | ______£® |
| ¢Ś£Ø20£© | ______£® | ______ | ______ |
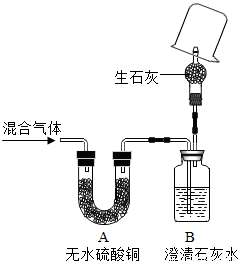
| ŹµŃé²½Öč | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ | |
| ¢Ł£Ø19£© | ŌŚ¼ā×ģµ¼¹Ü“¦µćČ¼ĘųĢ壬ŌŚ»šŃęÉĻ·½ÕÖŅ»Ö»øÉŌļµÄÉÕ±£® | ÉÕ±ÄŚ±ŚÓŠĖ®Öé | Ō»ģŗĻĘųĢåÖŠÓŠĒāĘų£® |
| ¢Ś£Ø20£© | ŌŚÉĻŹöÉÕ±ÖŠŃøĖŁµ¹ČėÉŁĮæ³ĪĒåŹÆ»ŅĖ®£¬Õńµ“£Ø»ņŌŚ»šŃęÉĻ·½ÕÖŅ»Ö»ÄŚ±ŚĶæÓŠ³ĪĒåŹÆ»ŅĖ®µÄÉÕ±£©£®£® | ³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬¼“²śÉś°×É«³Įµķ | Ō»ģŗĻĘųĢåÖŠÓŠŅ»Ńõ»ÆĢ¼ |


 Š”ѧɜ10·ÖÖÓæŚĖć²āŹŌ100·ÖĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓæŚĖć²āŹŌ100·ÖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
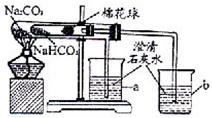 27”¢Ä³»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ŌŚŃ§Ļ°ĮĖ“æ¼ī£ØNa2CO3£©µÄŠŌÖŹŗó£¬ĮŖĻėµ½¼ŅÖŠÕōÖĘĀųĶ·Ź±³£ÓƵÄĪļÖŹŠ”ĖÕ“ņ£ØNaHCO3£©£¬ÓŚŹĒ²ÉÓĆŹŠŹŪµÄŠ”ĖÕ“ņѳʷ½ųŠŠĮĖČēĻĀŹµŃéĢ½¾æ£®
27”¢Ä³»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ŌŚŃ§Ļ°ĮĖ“æ¼ī£ØNa2CO3£©µÄŠŌÖŹŗó£¬ĮŖĻėµ½¼ŅÖŠÕōÖĘĀųĶ·Ź±³£ÓƵÄĪļÖŹŠ”ĖÕ“ņ£ØNaHCO3£©£¬ÓŚŹĒ²ÉÓĆŹŠŹŪµÄŠ”ĖÕ“ņѳʷ½ųŠŠĮĖČēĻĀŹµŃéĢ½¾æ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
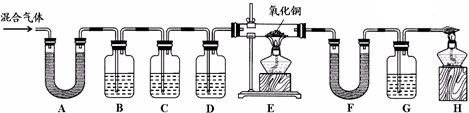
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ½ĖÕŹ”Ä£ÄāĢā ĢāŠĶ£ŗŹµŃéĢā
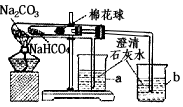
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗÕņ½ ĢāŠĶ£ŗĢīæÕĢā
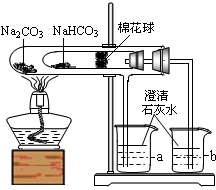
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2011ÄźĮÉÄžŹ”“óĮ¬ŹŠÖŠæ¼»Æѧ×ŌŹŹÓ¦Į·Ļ°£Ø¶ž£©£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
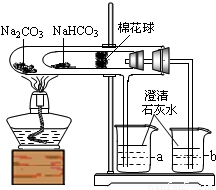
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com