���𰸡�
��������1��ϡ���������ᣬ�������������ڼ����Ҫ�������Ӧ��������ͼͬ��������ѡ���Լ���
��2����Ϊ������̼��ϡ�����Ӧ��������������Ȼ��Ӧ�������������Բ����ö�����̼������ϡ������������ƣ�
��3�����������غ㶨�ɿ�֪����Һ�������������������ӵĶ�����̼��������Ȼ����ݶ�����̼���������Ʒ�Ӧ�Ļ�ѧ����ʽ�����ö�����̼��������������������ʵ������������������������Һ�����ʵ�����������
��4����Ϊ���������������Һ���и�ʴ�ԣ�����ʹ��ʱҪ����С�ģ���Ҫմ���·���Ƥ���ϣ�
��5�����������ƺͶ�����̼�ķ�Ӧ���뵽��������Ҳ�ܺ�����������Һ��Ӧ��
����⣺��1����ɫʯ����Һ������ɫ��̪Һ��PH��ֽ����п�������Ȼ��ý�����������ͭ�����������Ȳ����Լ����������������̼�����������������������������ͭ����������þ�Ȳ����Լ��̼��ƣ���̼���Ƶ�̼���Σ�������ͭ�����Ȼ�ͭ�ȼ����������Ȼ����ȣ�
��2������������������ƿ�ռ���ƿ������̼���壬�ֱ������ϡ���������������Һ��������ƿ������Ե�������������Һ�������������еμ�ϡ������ᣨ���������ƻ��������������Ȼ��ƻ��Ȼ������Σ����������ݲ��������г�������������˵�����������������̼������Ӧ��
��3���⣺������̼������Ϊ12.2g-10g=2.2g
���������Ƶ�����Ϊx
CO
2+2NaOH�TNa
2CO
3+H
2O
44 80
2.2g x
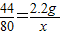
x=4g
��������������Һ�����ʵ���������Ϊ��

×100%=40%
��4��ʹ�����������������ҺʱҪ����С�ģ���Ϊ���������������Һ���и�ʴ�ԣ���Ҫմ���·���Ƥ���ϣ�
��5�����������ƺͶ�����̼�ķ�Ӧ���뵽��������Ҳ�ܺ�����������Һ��Ӧ������ʽΪ2NaOH+SO
2�TNa
2SO
3+H
2O��
�ʴ�Ϊ��
��1����ɫʯ����Һ��п
��2����������������ƿ�ռ���ƿ������̼���壬�ֱ������ϡ���������������Һ��������ƿ������Ե�������������Һ�����������еμ�ϡ���ᣬ�������ݲ�������˵�����������������̼������Ӧ
��3���𣺶�����̼������Ϊ2.2g������������Һ������������Ϊ40%��
��4�����������������Һ���и�ʴ��
��5��2NaOH+SO
2�TNa
2SO
3+H
2O��
������������һ���ۺ��Ե���Ŀ�������֪ʶ��϶֪࣬ʶ������ܴ�����ѣ�

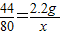 x=4g
x=4g ×100%=40%
×100%=40%

