




 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
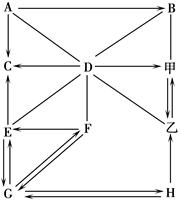
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
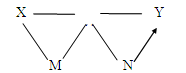
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ͭ�ͺ�ͭ��Ӳ�ȡ�����̻� | B���ռ���Һ�ͳ���ʯ��ˮ����������̼ |
| C��Ӳˮ����ˮ��������ˮ | D�������Ͷ�����̼����ȼ�ŵ�ľ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ô����ǵ�ľ�����ֿ��������� |
| B���÷���ˮ����Ӳˮ����ˮ |
| C����pH��ֽ�ⶨ��Һ������ |
| D������ɫʯ����Һ������Һ������������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ���� | ���� | �����ʵķ��� |
| A | ϡ���� | ͭ | ���� |
| B | �Ȼ�����Һ | ϡ���� | ����̼��ơ����� |
| C | ����������Һ | ����ͭ | �������ۡ����� |
| D | ������̼ | һ����̼ | ��ȼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ���� | �������� | ��ȥ���ʵķ��� |
| A�� | CO | H2 | ͨ��ˮ�У����� |
| B�� | NaCl | MgCl2 | �ܽ⡢���ˡ����� |
| C�� | KOH��Һ | K2CO3 | ��������ϡ���������ٲ������� |
| D�� | Cu(NO3)2��Һ | AgNO3 | ���������ͭ�ۣ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com