
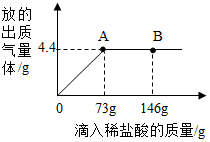
 �������Ĵ����Ʒ�����������������Ȼ��ƣ�ij��ѧ��ȤС���ͬѧͨ��ʵ�����ⶨij������Ʒ��̼���Ƶ�������������ȤС��ȡ15g ������Ʒ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
�������Ĵ����Ʒ�����������������Ȼ��ƣ�ij��ѧ��ȤС���ͬѧͨ��ʵ�����ⶨij������Ʒ��̼���Ƶ�������������ȤС��ȡ15g ������Ʒ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺���� ��1������̼����������ķ�Ӧ���ͼ�������������μ�ϡ������ͼ��A��ʱ���ձ�����Һ������ʣ�
��2��������̼���Ʒ�Ӧ�����������ʵ����������̼���Ƶ������������Ȼ��Ƶ���������̼���Ƶ����������������Ʒ��̼���Ƶ�����������
��3�����ݻ�������Ȼ��Ƶ������������Ȼ��Ƶ����������ǡ����ȫ��Ӧʱ��Һ�����ʵ�������
��� �⣺��1����ͼ���֪�����μ�ϡ������ͼ��A��ʱ��̼����ǡ����ϡ���ᷴӦ����Ӧ�����������73g���ձ�����Һ���������NaCl��
��2��ǡ����̼���Ʒ�Ӧ��ϡ���������ʵ�����Ϊ��73g��10%=7.3g��
��̼���Ƶ�����Ϊx�����ɵ��Ȼ��Ƶ�����Ϊy
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 73 117
x 7.3g y
$\frac{106}{x}=\frac{73}{7.3g}=\frac{117}{y}$
��ã�x=10.6g y=11.7g
�ô�����Ʒ��̼���Ƶ����������ǣ�$\frac{10.6g}{15g}��100%$��70.7%
��3��ǡ����ȫ��Ӧʱ����Һ�����ʵ�����Ϊ��15g-10.6g+11.7g=16.1g
�ʴ�Ϊ����1��NaCl����2����2���ô�����Ʒ��̼���Ƶ�����������70.7%����3��ǡ����ȫ��Ӧʱ����Һ�����ʵ�����Ϊ16.1g
���� ����Ҫ��ѧ����Ϥ��ȫ��Ӧ���ص㣬����ȷ������Һ�����ʵĹ�ϵ�������������ʵ������������м��㣬������ȷ���⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO��NH2��2 | B�� | Ca��OH��2 | C�� | Ca��H2PO4��2 | D�� | KCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������� | B�� | ���ݷ��� | C�� | ���ͻӷ� | D�� | ɽ�廬�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ͭ | B�� | ������þ | C�� | ̼��� | D�� | ��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO��NH2��2 | B�� | Ca��H2PO4��2 | C�� | K2CO3 | D�� | KNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������������⡢�Ҵ����� | B�� | ����������ء�����ϩ | ||
| C�� | ʯī�����顢������ | D�� | �֡��ɱ���Һ̬���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ҵ� �ƾ� C2H4O2 | B�� | ̼��� ̼� �� NH4��2CO3 | ||
| C�� | ������ ��ʯ�� CaO | D�� | ̼������ С�մ� NaHCO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com