
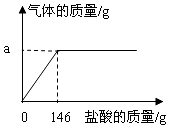
 ��ѧѧϰС��Ϊ�˷������������ĺ���������������ʵ���о���ȡ12g������10%��
��ѧѧϰС��Ϊ�˷������������ĺ���������������ʵ���о���ȡ12g������10%������ ��1����ͼʾ�ɵ������������
��2�������������ᷴӦ�Ļ�ѧ����ʽ����֪��������������������������ܹ��������������������������
��3��������Һ����Ӧ�������ʵ���������ȥ���������ʺ����ɵ������������
��� �⣺��1������ȫ��Ӧ��ȥ�����������ͼʾ�п��Կ�����146g���𰸣�146��
��2���������ᷴӦ����������Ϊx��
Fe+2HCl=FeCl2+H2��
56 73
x 146g��10%
$\frac{56}{x}=\frac{73}{146g��10%}$
x=11.2g
������������������Ϊ=$\frac{11.2g}{12g}$��100%=93.3%
��������������������Ϊ93.3%��
��3������������պ÷�Ӧ��ȫʱ������Һ����Ӧ�����ʵ���������ȥ���������ʺ����ɵ������������
�ʴ�Ϊ����1��146��
��2��93.3%����3����Һ��������������������ʣ�����ʵ����������Ի�Ҫ��ȥ���ʵ�������
���� ѧ�ῴ����ͼ����Ҫͬѧ�����յ�һ�ּ��ܣ�ץס��㣬ת�۵�����ߵ������ǹؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
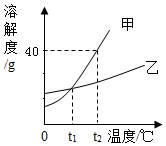
| A�� | t2��ʱ���ı�����Һ�����ʺ��ܼ�������֮��Ϊ2��5 | |
| B�� | t2��ʱ�ס��Ҹ�100g������Һ��t2�潵�µ�t1�棬��Һ��������� | |
| C�� | t1��ʱ���ס������ֱ�����Һ�����ʵ������������ | |
| D�� | ���к��������ң����Բ��ý��½ᾧ�ķ����ᴿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ���� | B�� | �Ȼ�����Һ | C�� | Ũ��ˮ | D�� | ����������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͬѧ��þ�����CuCl2��Һ��Ӧ���۲�������ݵĿ��� | |
| B�� | ��ͬѧ�����CuCl2��Һ�����ԣ�����pH��ֽ��� | |
| C�� | ��ͬѧ��п�����CuCl2��Һ��Ӧ���۲��Ƿ������ݲ��� | |
| D�� | ��ͬѧ�Ʋ�����������H2�����ռ������õ�ȼ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
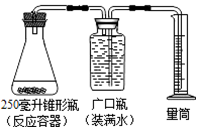 ijͬѧ������ͼװ�ã���һЩֲ�������������MnO2���������ֽ�H2O2��ȡ������ʵ���ȡ������������20%H2O2��Һ������34�ˣ���¼�������±���
ijͬѧ������ͼװ�ã���һЩֲ�������������MnO2���������ֽ�H2O2��ȡ������ʵ���ȡ������������20%H2O2��Һ������34�ˣ���¼�������±���| ��� | ֲ������ټ����� | �ռ����������������� | �ռ������ʱ�䣨���ӣ� |
| 1 | ��������ܲ�16�� | 25 | 5 |
| 2 | �����������16�� | 38 | 5 |
| 3 | ����������16�� | 80 | 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����̼ԭ�ӹ��ɵ��������ڴ����� | |
| B�� | ԭ����һ���������ӡ����ӡ����� | |
| C�� | NaCl�������ӹ��ɵģ�����HClҲ�������ӹ��ɵ� | |
| D�� | ��ԭ�Ӻ������ӵĺ˵����һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��ȡҺ����� | B�� | 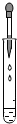 �μ�Һ�� | C�� | 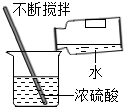 ϡ��Ũ���� | D�� | 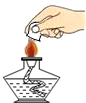 Ϩ��ƾ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʳ��� | B�� | �ƻ�ҩ | C�� | ʪ����ͭ | D�� | ��ˮɹ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com