
ĒėÄć²ĪÓėÅäÖĘŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄĒāŃõ»ÆÄĘČÜŅŗ£¬²¢½ųŠŠÓŠ¹Ų¼ĘĖć”£
£Ø1£©Š”ŗģĶ¬Ń§ŌŚŹµŃéŹŅÓūÅäÖĘ10%µÄĒāŃõ»ÆÄĘČÜŅŗ143g£¬Ēė»Ų“š£ŗ
¢ŁÅäÖĘ 10%µÄĒāŃõ»ÆÄĘČÜŅŗ143g£¬ŠčŅŖ40%µÄĒāŃõ»ÆÄĘČÜŅŗ£ØĆܶČĪŖ1.43g/cm3£© mL£¬¼ÓĖ® mL
£Ø½į¹ū±£ĮōŅ»Ī»Š”Źż£©”£
¢ŚŹµŃéÖŠ£¬±ŲŠčÓƵ½µÄŅĒĘ÷ÓŠ ”£
£Ø2£©½«Ņ»¶ØÖŹĮæµÄ¶žŃõ»ÆĢ¼ĶØČė 200g10%µÄĒāŃõ»ÆÄĘČÜŅŗÖŠ£¬Ē”ŗĆĶźČ«·“Ó¦£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬Éś³ÉµÄĢ¼ĖįÄʵÄÖŹĮæŹĒ g”£
£Ø1£©¢Ł25£»107.3£»¢ŚBDE£»£Ø2£©2NaOH+CO2£½Na2CO3+H2O£»26.5
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©ČÜŅŗÅäÖĘ¹ż³ĢÖŠ£¬ČÜÖŹµÄÖŹĮæ±£³Ö²»±ä£¬ÕāŹĒ¼ĘĖćµÄ»ł“”ŗĶĄķĀŪŅĄ¾Ż”£ÓÉÓŚ²ÉÓƵďĒĒāŃõ»ÆÄĘČÜŅŗ£¬ĖłŅŌĒāŃõ»ÆÄĘČÜŅŗŅ²ŅŖ¼ĘĖć³öĢå»ż£¬½ų¶ų½ųŠŠĮæČ””£ÅäÖĘ¹ż³ĢÖŠ£¬ÓÉÓŚ¶¼ŹĒČÜŅŗ£¬ĖłŅŌ²»ÓĆĶŠÅĢĢģĘ½£¬¶ųŹĒÓĆĮæĶ²£¬ÓƵ½ÉÕ±½ųŠŠĻ”ŹĶ£¬Ļ”ŹĶ¹ż³ĢÖŠŠčŅŖ²£Į§°ō½ųŠŠ½Į°č”£
¢ŁÅäÖĘ10%µÄĒāŃõ»ÆÄĘ143g£¬ĖłŠčŅŖµÄ40%µÄĒāŃõ»ÆÄĘČÜŅŗµÄÖŹĮæĪŖx£¬Ōņ
øł¾ŻČÜŅŗĻ”ŹĶĒ°ŗóČÜÖŹµÄÖŹĮæ±£³Ö²»±äæɵĆ
10%”Į143g£½40%”Įx
½āµĆx£½35.75g
¶ŌÓ¦µÄĢå»ż£½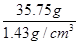 £½25cm3£½25mL
£½25cm3£½25mL
¼ÓČėµÄĖ®µÄÖŹĮæĪŖ143g£35.75g£½107.25g”Ö107.3g
ŌņĖ®¶ŌÓ¦µÄĢå»żĪŖ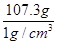 £½107.3cm3£½107.3mL
£½107.3cm3£½107.3mL
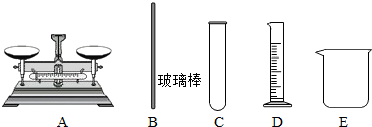
¢ŚÓÉÓŚĻ”ŹĶ¹ż³Ģ¶¼ŹĒŅŗĢ壬ĖłŅŌÓĆĮæĶ²¶ų²»ÓĆĶŠÅĢĢģĘ½£®Ļ”ŹĶŠčŅŖŌŚÉÕ±ÖŠ½ųŠŠ£¬Ķ¬Ź±ŠčŅŖ²£Į§°ō½Į°č£®Ņņ“ĖÓƵ½µÄŅĒĘ÷ĪŖBDE”£
£Ø2£©¶žŃõ»ÆĢ¼ÓėĒāŃõ»ÆÄĘĒ”ŗĆ·“Ó¦ÓŠČżÖÖæÉÄÜ£ŗĒāŃõ»ÆÄĘĶźČ«×Ŗ»ÆĪŖĢ¼ĖįÄĘ£»ĒāŃõ»ÆÄĘ×Ŗ»ÆĪŖĢ¼ĖįĒāÄĘŗĶĢ¼ĖįÄĘ£»ĒāŃõ»ÆÄĘ×Ŗ»ÆĪŖĢ¼ĖįĒāÄĘ£®¶ųĢāÄæŅŖĒó¼ĘĖćĢ¼ĖįÄʵÄÖŹĮ棬ĖłŅŌŹĒ¼ŁÉčֻɜ³ÉĮĖĢ¼ĖįÄĘ£¬ĘäĖūĒéæö²»æ¼ĀĒ”£
¶ŌÓ¦µÄ·“Ó¦·½³ĢŹ½ĪŖ2NaOH+CO2£½Na2CO3+H2O
Éč200g10%µÄĒāŃõ»ÆÄĘĶźČ«×Ŗ»ÆĪŖĢ¼ĖįÄĘŹ±£¬Éś³ÉµÄĢ¼ĖįÄʵÄÖŹĮæĪŖy
2NaOH+CO2£½Na2CO3+H2O
80 106
200g”Į10% y
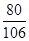 £½
£½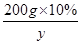
½āµĆy£½26.5g
æ¼µć£ŗæ¼²éŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄČÜŅŗµÄÅäÖĘ


 Ńō¹āæ¼³”µ„ŌŖ²āŹŌ¾ķĻµĮŠ“š°ø
Ńō¹āæ¼³”µ„ŌŖ²āŹŌ¾ķĻµĮŠ“š°ø ĆūŠ£ĮŖĆĖ³å“Ģ¾ķĻµĮŠ“š°ø
ĆūŠ£ĮŖĆĖ³å“Ģ¾ķĻµĮŠ“š°ø ĆūŠ£Ģį·ÖŅ»¾ķĶØĻµĮŠ“š°ø
ĆūŠ£Ģį·ÖŅ»¾ķĶØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĒėÄć²ĪÓėÅäÖĘŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄĒāŃõ»ÆÄĘČÜŅŗ£¬²¢½ųŠŠÓŠ¹Ų¼ĘĖć”£
£Ø1£©Š”ŗģĶ¬Ń§ŌŚ ŹµŃéŹŅÓūÅäÖĘ 10%µÄĒāŃõ»ÆÄĘČÜŅŗ 143g£¬Ēė»Ų“š£ŗ
ŹµŃéŹŅÓūÅäÖĘ 10%µÄĒāŃõ»ÆÄĘČÜŅŗ 143g£¬Ēė»Ų“š£ŗ
3
¢Ł ÅäÖĘ 10%µÄĒāŃõ»ÆÄĘČÜŅŗ 143g£¬ŠčŅŖ 40%µÄĒāŃõ»ÆÄĘČÜŅŗ£ØĆܶČĪŖ 1.43g/cm £©_____
mL£¬¼ÓĖ® mL(½į¹ū±£ĮōŅ»Ī»Š”Źż)”£
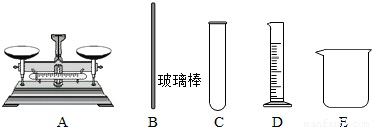
¢Ś ŹµŃéÖŠ£¬±ŲŠčÓƵ½µÄŅĒĘ÷ÓŠ ”£
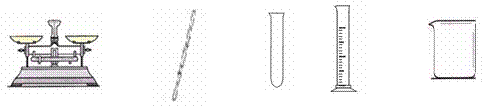
²£Į§°ō
A B C D E
£Ø2£©½«Ņ»¶ØÖŹĮæµÄ¶žŃõ»ÆĢ¼ĶØČė 200g10%µÄĒāŃõ»ÆÄĘČÜŅŗÖŠ£¬Ē”ŗĆĶźČ«·“Ó¦£¬·“Ó¦µÄ»Æѧ
·½³ĢŹ½ĪŖ £¬Éś³ÉµÄĢ¼ĖįÄʵÄÖŹĮæŹĒ g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĒėÄć²ĪÓėÅäÖĘŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄĒāŃõ»ÆÄĘČÜŅŗ£¬²¢½ųŠŠÓŠ¹Ų¼ĘĖć”£
£Ø1£©Š”ŗģĶ¬Ń§ŌŚŹµŃéŹŅÓūÅäÖĘ10%µÄĒāŃõ»ÆÄĘČÜŅŗ143g£¬Ēė»Ų“š£ŗ
¢ŁÅäÖĘ 10%µÄĒāŃõ»ÆÄĘČÜŅŗ143g£¬ŠčŅŖ40%µÄĒāŃõ»ÆÄĘČÜŅŗ£ØĆܶČĪŖ1.43g/cm3£© mL£¬¼ÓĖ® mL
£Ø½į¹ū±£ĮōŅ»Ī»Š”Źż£©”£
¢ŚŹµŃéÖŠ£¬±ŲŠčÓƵ½µÄŅĒĘ÷ÓŠ ”£
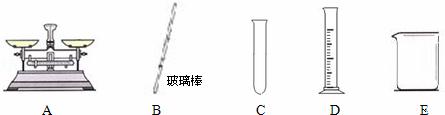
£Ø2£©½«Ņ»¶ØÖŹĮæµÄ¶žŃõ»ÆĢ¼ĶØČė 200g10%µÄĒāŃõ»ÆÄĘČÜŅŗÖŠ£¬Ē”ŗĆĶźČ«·“Ó¦£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬Éś³ÉµÄĢ¼ĖįÄʵÄÖŹĮæŹĒ g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ¹ć¶«Ź”¹ćÖŻŹŠŗ£ÖéĒų¾ÅÄź¼¶£ØĻĀ£©×ŪŗĻĮ·Ļ°»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com