
��ѧ��ȤС���ij��ҵ��ˮ������H2SO4��Na2SO4����H2SO4�ĺ������вⶨ���ס�����ͬѧ�������ͬ�IJⶨ������
��1����ͬѧ������кͷ�
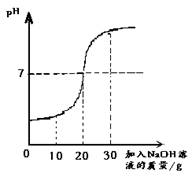
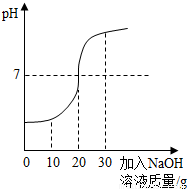
ȡ60g��ˮ���ձ��У�����������������Ϊ10%��NaOH��Һ����Ӧ�Ļ�ѧ����ʽΪ��H2SO4+2NaOH==Na2SO4+ 2H2O������Ӧ������ҺpH�仯����ͼ�� ��60g��ˮ��H2SO4��������
��2����ͬѧ������������
����BaCl2��Һ����NaOH��Һ�ⶨ��ˮ�е�H2SO4�ĺ�������Ӧ�Ļ�ѧ����ʽΪ��H2SO4+ BaCl2==BaSO4��+2HCl��������Ϊ����� ���ƫ�ߡ�����ƫ�͡��������䡱�������� ��
(1) 2.45g
��2��ƫ�ߣ�1�֣� BaCl2��ҺҲ�����ˮ�е�Na2SO4��Ӧ����2�֣�
����������1����PH=7ʱ����Һ�����ԣ�˵������ǡ����ȫ��Ӧ����ʱ�����NaOH��Һ������Ϊ20�ˡ�
��:���ˮ�����������Ϊx
H2SO4+2NaOH=Na2SO4+2H2O
98 80
x 20g��10%
98/80=X/20g��10% ��2�֣�
X=2.45g ��1�֣�
�𣺷�ˮ�����������Ϊ2.45g
��2��ƫ�ߣ���Ϊ BaCl2��ҺҲ�����ˮ�е�Na2SO4��Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
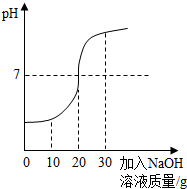 ��2012?���£���ѧ��ȤС���ij��ҵ��ˮ������H2SO4��Na2SO4����H2SO4�ĺ������вⶨ���ס�����ͬѧ�������ͬ�IJⶨ������
��2012?���£���ѧ��ȤС���ij��ҵ��ˮ������H2SO4��Na2SO4����H2SO4�ĺ������вⶨ���ס�����ͬѧ�������ͬ�IJⶨ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ��������¾�����ѧ���������� ���ͣ�������
��ѧ��ȤС���ij��ҵ��ˮ������H2SO4��Na2SO4����H2SO4�ĺ������вⶨ���ס�����ͬѧ�������ͬ�IJⶨ������
��1����ͬѧ������кͷ�
ȡ60g��ˮ���ձ��У�����������������Ϊ10%��NaOH��Һ����Ӧ�Ļ�ѧ����ʽΪ��H2SO4+2NaOH==Na2SO4+ 2H2O������Ӧ������ҺpH�仯����ͼ����60g��ˮ��H2SO4��������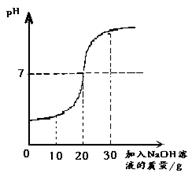
��2����ͬѧ������������
����BaCl2��Һ����NaOH��Һ�ⶨ��ˮ�е�H2SO4�ĺ�������Ӧ�Ļ�ѧ����ʽΪ��H2SO4+ BaCl2==BaSO4��+2HCl��������Ϊ����� ���ƫ�ߡ�����ƫ�͡��������䡱�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
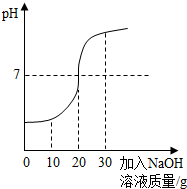 ��ѧ��ȤС���ij��ҵ��ˮ������H2SO4��Na2SO4����H2SO4�ĺ������вⶨ���ס�����ͬѧ�������ͬ�IJⶨ������
��ѧ��ȤС���ij��ҵ��ˮ������H2SO4��Na2SO4����H2SO4�ĺ������вⶨ���ס�����ͬѧ�������ͬ�IJⶨ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�긣��ʡ�������п���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com