
�ش��������⣬д���йػ�ѧ����ʽ��
��1��������ͭ��Һ�м����Ȼ�����Һ���۲쵽�������� ����Ӧ�Ļ�ѧ����ʽΪ ��
��2��þ�ڿ�����ȼ����������þ�͵���þ�����е�Ϊ��3�ۣ�������þ��ˮ��Ӧ����������þ�Ͱ�����NH3����
��д������þ�Ļ�ѧʽ ��
����֪����þ��һ�ֻ���ɫ�Ĺ��壮����þ�ڿ�����ȼ�յ�ʵ������֪�������������£�þ����������е� ���ϣ������� ��
��д������þ��ˮ��Ӧ�Ļ�ѧ����ʽ ��
�� g����þ�к��� 24g þԪ�أ���ȷ�� 0.1g����
��1��CuSO4+BaCl2��BaSO4��+CuCl2����2����Mg3N2����O2��������������ǰ�ɫ�ģ�˵��������MgO�϶࣬Mg3N2���٣���Mg3N2+6H2O��3Mg(OH)2��+2NH3������33.3g
���������������1������ͭ���Ȼ�����Ӧ�������ᱵ�������Ȼ�ͭ���ݴ��жϷ�Ӧ�������д����ʽ������ͭ���Ȼ�����Ӧ�������ᱵ�������Ȼ�ͭ�����Զ���Ϻ��۲쵽�а�ɫ�������ɣ�����ʽ�ǣ�CuSO4+BaCl2��BaSO4��+CuCl2��
��2���پݻ�ѧʽ����д���н�𡣵���þ�е�Ϊ��3�ۣ���Ϊ��2�ۣ���������ǰ�����ۺ��ݽ��淨��д���ʵĻ�ѧʽΪ��Mg3N2��
��þ�ڿ�����ȼ��ʱ������������ǰ�ɫ�ģ��ݴ˷������þ�ڿ�����ȼ��ʱ������������ǰ�ɫ�ģ�������þ��һ�ֻ���ɫ�Ĺ��壬����þ�ڿ�����ȼ��ʱ������������Ӧ��
�۾ݷ�Ӧ���������д����ʽ����ע����ƽ������þ��ˮ��Ӧ����������þ�Ͱ�����NH3��������ʽ�ǣ�Mg3N2+6H2O��3Mg(OH)2��+2NH3����
�ܾݻ�������Ԫ�ص������������м��㡣���ݡ�������ijԪ�ص��������û�������������û������и�Ԫ�ص�������������֪������þ�������ǣ�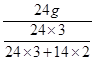 ��33.3g.
��33.3g.
���㣺�����εĻ�ѧ���ʣ���ѧʽ����д�����壻��������ijԪ�ص��������㣻��ѧ����ʽ��д


 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ƹ��屩¶�ڿ������ױ��ʣ��������� ���û�ѧ����ʽ��ʾ����ʵ��������һƿ�������ƹ��壬Ҫ�������Ƿ��Ѿ����ʣ���ȡ�����������Թ��У�Ȼ����� �����顣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ҫѧϰ�û�ѧ���ڿ�����Ҫ�����������������ص�����ÿ��ñʼǡ�����������ͬѧ�Ŀ��ñʼǣ��������ؿո���������
��1�������ʵ��Ļ�ѧ���ţ�
��2�������� ��
��þ�ڿ�����ȼ�����ɵ���Ҫ������Ļ�ѧʽ ��
������������Ԫ�صĻ��ϼ� ��
��笠����� ��
�ݵؿ��к������Ľ���Ԫ�ص�����������������γɵĻ�����Ļ�ѧʽ ��
���Ȼ������� ���ɵģ�
��2��˵�����֡�2���ĺ��壺
2O2-��ǰ��ġ�2����ʾ �����Ͻǵġ�2����ʾ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ԫ�����ڱ���ѧϰ���о���ѧ����Ҫ���ߡ���ͼ��Ԫ�����ڱ���һ���֣�
| �� ���� | ��A | | 0 | |||||
| 1 | 1 H 1.008 | ��A | ��A | ��A | ��A | ��A | ��A | 2 He 4.003 |
| 2 | 3 Li 6.941 | 4 Be 9.012 | 5 B 10.8l | 6 C 12.01 | 7 N 14.0l | 8 O 16.00 | 9 F 19.00 | 10 Ne 20.8l |
| 3 | 11 Na 22.99 | 12 Mg 24.31 | 13 Al 26.98 | 14 Si 28.09 | 15 P 30.97 | 16 S 32.06 | 17 Cl 35.45 | 18 Ar 39.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ�ط��Ż�ѧʽ��ʾ
(1) 2����ԭ��___________��
(2) ����______________��
(3) 5��������__________��
(4) ���ֶ�����̼��ѧ���ʵ���С����________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ȷ�Ļ�ѧ������գ�
��1��1����ԭ�� ��
��2��2��̼����� ��
��3���ĸ��������� ��
��4�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д�����л�ѧʽ�����ֵĺ��壺
��1��3��������������___________��
��2��Mg2+��2�����___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������ѧ���ӻ�ѧ����������¿γ̸ĸ�����������ͨ����һ�껯ѧ��ѧϰ�������û�ѧ֪ʶ���������е�����������г��ֵ����⣺
��1��Ϊ����ʹ���ǰ��Ե���˳��ڱ��棬����һ���ǽ���˷����չ��У����ϸǺ����ڸǵ���Χ��������ˮ��������Ŀ���� �Է���˱��ʣ�
��2����¯��������Ҫԭ���У�����ʯ����̿��ʯ��ʯ�ȣ���¯�ڷ�������Ҫ��ѧ��Ӧ�У�
�ٽ�̿ȼ��Ϊ�����ṩ���� ��
���Խ�̿Ϊԭ����ȡ��ԭ�� ��
�۸������û�ԭ����ԭ����ʯ���Դ�����Ϊ����������� ��
��3��ij�ؾ����鷢���������д���ʹ�ú���ú��Ϊȼ����������������ŷŴ����Ķ�����̼���������꣬������������ĸĽ���ʩ������ú������������ʯ�Ҽ����������ɵĶ���������ԭ�����û�ѧ����ʽ��ʾΪ ��
��4��Ҫϴȥ��ˮ�õ������ڱ��ϵ�ˮ��[��Ҫ��CaCO3��Mg(OH)2]���ɼ�������ȥ����صĻ�ѧ����ʽΪ��CaCO3+2CH3COOH=(CH3COO)2Ca+H2O+CO2����Mg(OH)2+2CH3COOH=(CH3COO)2Mg+2H2O��������Ĵ���ܹ�������Ϊ�ᷢ����Ӧ___ ______________________________��ʹ������ʴ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�û�ѧ������գ�
��1�������۵���Ԫ������ ����2��2��þԭ���� ������3��5����������� ����
��4��ʵ�����ù�����������������������Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com