A”¢B”¢C”¢D”¢EŹĒ³õÖŠ»Æѧ֊µÄĪåÖÖ³£¼ūĪļÖŹ£®C”¢DŹĒµ„ÖŹ£¬CĪŖ×ĻŗģÉ«¹ĢĢ壬DŹĒĆܶČ×īŠ”µÄĘųĢ壬BµÄÅØČÜŅŗ³£ÓĆ×÷øÉŌļ¼Į£®ŅŃÖŖFeŗĶA”¢BµÄĖ®ČÜŅŗ·Ö±šÄÜ·¢Éś·“Ó¦£ŗ
¢ŁFe+A”śC+E£»¢ŚFe+B”śD+E
£Ø1£©Š“³öA”¢B”¢CµÄ»ÆѧŹ½£ŗA
CuSO4
CuSO4
B
H2SO4
H2SO4
C
Cu
Cu
£Ø2£©ĻÖÓŠFeŗĶA”¢CČżÖÖ¹ĢĢå×é³ÉµÄ»ģŗĻĪļ£¬Ä³»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§ĻėĶعżŹµŃé²ā¶ØøĆ»ģŗĻĪļÖŠCĪļÖŹµÄÖŹĮæ·ÖŹż£®
”¾Éč¼ĘŹµŃé”æČēĶ¼ĖłŹ¾
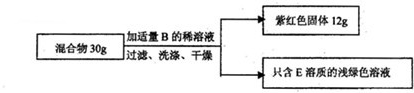
”¾Źż¾Ż“¦Ąķ”æ
¢ŁŠ”øÕøł¾ŻŹż¾Ż¼ĘĖć³ö»ģŗĻĪļÖŹÖŠCĪļÖŹµÄÖŹĮæ·ÖŹżĪŖ£ŗ
”Į100%£¬Š”ĒæČĻĪŖŹż¾Ż“¦Ąķ²»ÕżČ·£¬ŌŅņŹĒ
12g×ĻŗģÉ«¹ĢĢåÖŠ°üŗ¬ÓŠĢśŗĶĮņĖįĶ·“Ӧɜ³ÉµÄĶ£ØĢśŗĶĮņĖįĶ·“Ӧɜ³ÉĮĖĶ»ņ12g×ĻŗģÉ«¹ĢĢ壬²»Ö»ŹĒŌĄ“µÄCĪļÖŹ£¬»ņ12g×ĻŗģÉ«¹ĢĢåµÄÖŹĮæ“óÓŚŌĄ“CĪļÖŹµÄÖŹĮ棩£®
12g×ĻŗģÉ«¹ĢĢåÖŠ°üŗ¬ÓŠĢśŗĶĮņĖįĶ·“Ӧɜ³ÉµÄĶ£ØĢśŗĶĮņĖįĶ·“Ӧɜ³ÉĮĖĶ»ņ12g×ĻŗģÉ«¹ĢĢ壬²»Ö»ŹĒŌĄ“µÄCĪļÖŹ£¬»ņ12g×ĻŗģÉ«¹ĢĢåµÄÖŹĮæ“óÓŚŌĄ“CĪļÖŹµÄÖŹĮ棩£®
¢ŚĖūĆĒ¾¹żČĻÕę·ÖĪöŗ󣬷¢ĻÖ»¹Č±ÉŁ±ŲŅŖµÄŹµŃ鏿¾Ż£¬¾¹żÖŲŠĀŹŌŃ飬²¢ÓĆĢģĘ½³Ę³ö·“Ó¦Ē°ŗó×ÜÖŹĮæ¼õÉŁĮĖ0£¬1g£¬“Ó¶ųĖć³ö»ģŗĻĪļÖŠCĪļÖŹµÄÖŹĮæ·ÖŹżĪŖ
18.7%
18.7%
£®
”¾ŹµŃé·“Ė¼”æ
Ķ¬Ń§ĆĒ¶ŌøĆ»ģŗĻĪļµÄ³É·Ö¾¹żČĻÕę·ÖĪöŗó·¢ĻÖ£¬Ö»øł¾ŻĪļÖŹµÄĪļĄķŠŌÖŹŅ²Äܲā¶ØCĪļÖŹµÄÖŹĮæ·ÖŹż£¬ŹŌŠ“³ö¼ņĆ÷µÄŹŌŃé·½·Ø
½«ĪļÖŹČÜÓŚĖ®£¬¹żĀĖ£¬Ļ“µÓŗęøÉ£¬³ĘµĆĀĖŌüÖŹĮ森ÓĆ“ÅĢśĪüŹÕĀĖŌü£¬³ĘµĆŹ£ÓąĪļÖŹµÄÖŹĮ漓ĶµÄÖŹĮ棬ÓĆĶµÄÖŹĮæ³żŅŌ×ÜÖŹĮ漓µĆĶµÄÖŹĮæ·ÖŹż£®
½«ĪļÖŹČÜÓŚĖ®£¬¹żĀĖ£¬Ļ“µÓŗęøÉ£¬³ĘµĆĀĖŌüÖŹĮ森ÓĆ“ÅĢśĪüŹÕĀĖŌü£¬³ĘµĆŹ£ÓąĪļÖŹµÄÖŹĮ漓ĶµÄÖŹĮ棬ÓĆĶµÄÖŹĮæ³żŅŌ×ÜÖŹĮ漓µĆĶµÄÖŹĮæ·ÖŹż£®
£®





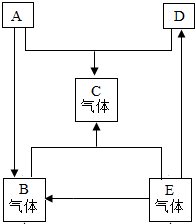 ŅŃÖŖA”¢B”¢C”¢D”¢EŹĒ³õÖŠ»Æѧ³£¼ūµÄĪåÖÖĪļÖŹ£¬ĘäÖŠA”¢DŹĒŗŚÉ«¹ĢĢ壬AĪŖŗŚÉ«¹ĢĢåµ„ÖŹ£¬DĪŖŗŚÉ«¹ĢĢåŃõ»ÆĪļ£¬ĒŅĘäÖŠŗ¬ÓŠČĖĄąŹ¹ÓĆ×ī¹ć·ŗµÄ½šŹōŌŖĖŲ£¬B”¢C”¢EŹĒĪŽÉ«ĘųĢ壬£®ĖüĆĒŌŚŅ»¶ØĢõ¼žĻĀµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ØĘäĖū·“Ó¦ĪļŗĶÉś³ÉĪļŅŃĀŌČ„£©£®
ŅŃÖŖA”¢B”¢C”¢D”¢EŹĒ³õÖŠ»Æѧ³£¼ūµÄĪåÖÖĪļÖŹ£¬ĘäÖŠA”¢DŹĒŗŚÉ«¹ĢĢ壬AĪŖŗŚÉ«¹ĢĢåµ„ÖŹ£¬DĪŖŗŚÉ«¹ĢĢåŃõ»ÆĪļ£¬ĒŅĘäÖŠŗ¬ÓŠČĖĄąŹ¹ÓĆ×ī¹ć·ŗµÄ½šŹōŌŖĖŲ£¬B”¢C”¢EŹĒĪŽÉ«ĘųĢ壬£®ĖüĆĒŌŚŅ»¶ØĢõ¼žĻĀµÄ×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ØĘäĖū·“Ó¦ĪļŗĶÉś³ÉĪļŅŃĀŌČ„£©£® 23”¢ŅŃÖŖA”¢B”¢C”¢D”¢EŹĒ³õÖŠ»ÆѧĄļ³£¼ūµÄĪåÖÖĪļÖŹ£¬ĖüĆĒŌŚŅ»¶ØĢõ¼žĻĀÄÜ·¢ÉśČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ£¬ĘäÖŠ·“Ó¦¢ŁŹĒø“·Ö½ā·“Ó¦£¬EŹĒŌģ³ÉĪĀŹŅŠ§Ó¦µÄÖ÷ŅŖĘųĢ壮
23”¢ŅŃÖŖA”¢B”¢C”¢D”¢EŹĒ³õÖŠ»ÆѧĄļ³£¼ūµÄĪåÖÖĪļÖŹ£¬ĖüĆĒŌŚŅ»¶ØĢõ¼žĻĀÄÜ·¢ÉśČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ£¬ĘäÖŠ·“Ó¦¢ŁŹĒø“·Ö½ā·“Ó¦£¬EŹĒŌģ³ÉĪĀŹŅŠ§Ó¦µÄÖ÷ŅŖĘųĢ壮 A”¢B”¢C”¢D”¢EŹĒ³£¼ūĪļÖŹ£¬ĘäÖŠBŗĶEŹĒæÕĘųÖŠĮ½ÖÖĘųĢ壬CŹĒĢśŠāÖ÷ŅŖ³É·Ö£®ČēĶ¼ĖłŹ¾£¬Ö±Ļß±ķŹ¾Ļą»„¼äÄܹ»·“Ó¦£¬¼żĶ·±ķŹ¾×Ŗ»ÆµÄ·½Ļņ£®
A”¢B”¢C”¢D”¢EŹĒ³£¼ūĪļÖŹ£¬ĘäÖŠBŗĶEŹĒæÕĘųÖŠĮ½ÖÖĘųĢ壬CŹĒĢśŠāÖ÷ŅŖ³É·Ö£®ČēĶ¼ĖłŹ¾£¬Ö±Ļß±ķŹ¾Ļą»„¼äÄܹ»·“Ó¦£¬¼żĶ·±ķŹ¾×Ŗ»ÆµÄ·½Ļņ£® £Ø2013?ŠģÖŻ¶žÄ££©A”¢B”¢C”¢D”¢EŹĒ³õÖŠ»Æѧ֊³£¼ūµÄ²»Ķ¬Ąą±šµÄĪļÖŹ£ØĪļÖŹ°“µ„ÖŹ”¢Ńõ»ÆĪļ”¢Ėį”¢¼ī”¢ŃĪ·ÖĄą£©£®ŅŃÖŖAŹĒµ„ÖŹ£»CŹĒŗģ×ŲÉ«¹ĢĢ壻EŹĒĖ®ČÜŅŗæÉŹ¹·ÓĢŖŹŌŅŗ±äĪŖŗģÉ«µÄŃĪ£®Ķ¼ÖŠ”°-”±±ķŹ¾ĻąĮ¬µÄĪļÖŹĮ½Į½Ö®¼äæÉŅŌ·¢Éś·“Ó¦£¬”°”ś”±±ķŹ¾ÓÉijŅ»ĪļÖŹæÉÖʵĆĮķŅ»ĪļÖŹ£Ø²æ·Ö·“Ó¦Īļ”¢Éś³ÉĪļ¼°·“Ó¦Ģõ¼žŅŃĀŌČ„£©£®»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø2013?ŠģÖŻ¶žÄ££©A”¢B”¢C”¢D”¢EŹĒ³õÖŠ»Æѧ֊³£¼ūµÄ²»Ķ¬Ąą±šµÄĪļÖŹ£ØĪļÖŹ°“µ„ÖŹ”¢Ńõ»ÆĪļ”¢Ėį”¢¼ī”¢ŃĪ·ÖĄą£©£®ŅŃÖŖAŹĒµ„ÖŹ£»CŹĒŗģ×ŲÉ«¹ĢĢ壻EŹĒĖ®ČÜŅŗæÉŹ¹·ÓĢŖŹŌŅŗ±äĪŖŗģÉ«µÄŃĪ£®Ķ¼ÖŠ”°-”±±ķŹ¾ĻąĮ¬µÄĪļÖŹĮ½Į½Ö®¼äæÉŅŌ·¢Éś·“Ó¦£¬”°”ś”±±ķŹ¾ÓÉijŅ»ĪļÖŹæÉÖʵĆĮķŅ»ĪļÖŹ£Ø²æ·Ö·“Ó¦Īļ”¢Éś³ÉĪļ¼°·“Ó¦Ģõ¼žŅŃĀŌČ„£©£®»Ų“šĻĀĮŠĪŹĢā£ŗ A”¢B”¢C”¢D”¢EŹĒ³õÖŠ»Æѧ֊³£¼ūµÄ5ÖÖĪļÖŹ£¬ĖüĆĒ¶¼ŗ¬ÓŠŅ»ÖÖĻąĶ¬µÄŌŖĖŲ£¬ČēĶ¼±ķŹ¾ø÷ĪļÖŹÖ®¼äµÄ×Ŗ»Æ¹ŲĻµ£®ĘäÖŠ£¬AĪŖŹ³ŃĪµÄÖ÷ŅŖ³É·Ö£¬BÖŠŗ¬±µŌŖĖŲ£¬D”¢EµÄČÜŅŗ¶¼ÓŠŃÕÉ«£¬
A”¢B”¢C”¢D”¢EŹĒ³õÖŠ»Æѧ֊³£¼ūµÄ5ÖÖĪļÖŹ£¬ĖüĆĒ¶¼ŗ¬ÓŠŅ»ÖÖĻąĶ¬µÄŌŖĖŲ£¬ČēĶ¼±ķŹ¾ø÷ĪļÖŹÖ®¼äµÄ×Ŗ»Æ¹ŲĻµ£®ĘäÖŠ£¬AĪŖŹ³ŃĪµÄÖ÷ŅŖ³É·Ö£¬BÖŠŗ¬±µŌŖĖŲ£¬D”¢EµÄČÜŅŗ¶¼ÓŠŃÕÉ«£¬