Ϊ���Ƴ��л�������������������������̬��Ӧ������½��ʹ����Ȼ�����Ҵ����͵������Դ��
��1������������ȼ�ϵĸ����������£�

�������йؼ���ȼ�ϸ��µ����ɣ���ȷ����
��
A����Ȼ���ǿ���������Դ
B������ȼ�ϵ������ʸ���
C������úȼ�չ����в�������Ⱦ��
����Ȼ������Ҫ�ɷ��Ǽ��飩��Һ��ʯ��������Ҫ�ɷ��DZ���Ͷ��飩��̼Ԫ�ص����������ֱ�ԼΪ75%��82.3%��ͨ�������ݷ�������ͬ�����£�����Ȼ����ȼ�ϱ�Һ��ʯ����������������
��
�ۼ����ڿ�������ȫȼ�յĻ�ѧ����ʽΪ
��
��2�����½����͵�������վ����Ȼ���辭�����ӡ���������ζ������ѹ�ȴ�����������͵�������У��÷��ӵĹ۵������Ȼ����ѹ����������͵�ԭ��
�����������������������������
��
��3��Ϊ��һ�������������������ؽ����ƹ��Ҵ����͵�ʹ�ã���ν�Ҵ��������������м��������Ҵ���϶��ɵ�һ��ȼ�ϣ�
���Ҵ���������
��ѡ����������������
�ڴӻ��������ĽǶȿ�������ȼ�������������
����д��ĸ��ţ���
A������ B��ú C���Ҵ����� D��ú������Ҫ��CO��
��4��Ϊ�ⶨԭú�еĺ�̼����Ҫ�ô���������С��ͬѧ��ʵ�����������Ĺ����з��֣��ռ�������ռ����ƿ�ݻ���60%������ռ40%��ʱ����ʹ�����ǵ�ľ����ȼ����ô��ʹ�����ǵ�ľ����ȼ������Ũ�ȵ����ֵ�Ƕ����أ�С���Դ�չ��̽����
��һ��ʵ�飺ȡ5ֻ����ƿ�����Ϊ�١��ڡ��ۡ��ܡ��ݣ��ֱ�װ�������ݻ�10%��20%��30%��40%��50%��ˮ������ˮ���ռ�������ǡ�ð�5ֻ����ƿ�е�ˮ��ȥ���������ǵ�ľ�����β���١��ݺ�ƿ�У���¼ʵ������
С����ǰһ��ʵ��Ļ����������˵ڶ���͵�����ʵ�飬����ʵ������ݺ�������±���
| ��� |
һ |
�� |
�� |
| ����ƿ��� |
�� |
�� |
�� |
�� |
�� |
�� |
�� |
�� |
�� |
�� |
�� |
| ƿ��O2ռ�ݻ������������%�� |
l0 |
20 |
30 |
40 |
50 |
3l |
33 |
35 |
37 |
39 |
34 |
| ������ľ����״�� |
�� |
�� |
���� |
��ȼ |
��ȼ |
���� |
���� |
��ȼ |
��ȼ |
��ȼ |
���� |
�����ʵ��ش��������⣺
��ʹ�ô����ǵ�ľ�����������ķ����Ƿ�ɿ���
����ǡ���'����
���ռ�������ռ�ݻ�������������Ϊ
%ʱ����ʹ�����ǵ�ľ����ȼ����ʱ����ƿ���������������������
%��������������������
�۲�ȡ����ֵ����̽���ܼ���ʵ����������磺��һ��ʵ�������ۡ��ܵı��˳�����ʵ�飬����ȷ����һ��ʵ���ռ�������ռ�ݻ����������Ӧ��30%��40%֮�䣬�Ӷ�ʡȥ���Ϊ�١��ڡ��ݵ�ʵ�飮ͬ�����ڶ���ʵ�����ʡȥ��ʵ����Ϊ
��
��5���������ռ��������ٽ���ʵ�飬����������ȷ�����أ����С����ͬѧ�Ǿ�����������װ�ý��вⶨ����ͼ1����
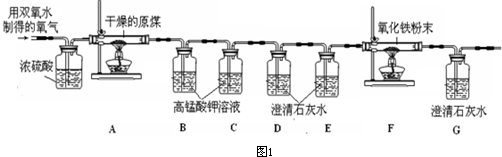
���ϣ��ٸ���ԭúȼ�ղ�����������Ҫ��CO
2����ͬʱ��������SO
2��CO��
��SO
2���������أ�KMnO
4����Ӧ��ʹ��Һ�Ϻ�ɫ��ȥ��Ҳ��ʹ����ʯ��ˮ����ǣ���CO
2��CO������ʹ�Ϻ�ɫ���������Һ��ɫ�������װ��ͼ2�ش�
��Ũ�����������
��ѡ��A��B���� A���������� B����ȥ�����м�������
����д��Bװ�ú�Cװ�õ����ã�Bװ�õ�����
��Cװ�õ�����
��
������B��Cװ���иijɳ���ʯ��ˮ��D��Eװ���иijɸ��������Һ�����ԭú��̼Ԫ�ص���������
���ƫ��ƫС������
��F�з�Ӧ�Ļ�ѧ����ʽ
��
��β��Ӧ��δ�����
��
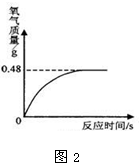
��6������������Һ���ڱ������Ȼ�ֽ⣬ʹ����������������С��Ϊȷ��ʵ��ʱ˫��ˮ��������С����ͬѧ�����ȶ�ʵ�����ṩ�Ĺ���������Һ�������������������ⶨ������ȡ������Һ51g�����������������̣����������������뷴Ӧʱ��Ĺ�ϵ��ͼ2��ʾ��
����ȫ��Ӧ����������������Ϊ
g��
�ڼ���ù���������Һ�����ʵ�����������










