


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
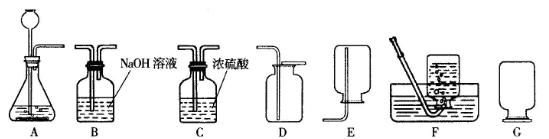
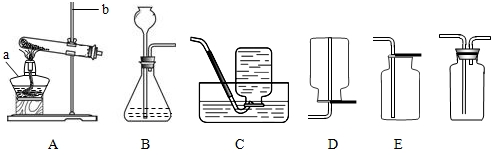
(1)(6��)��������������壬������ˮ��������Ϊδ����������Դ���Ǻ��칤ҵ�ĸ���ȼ�ϣ�Ҳ�ǻ�����������Ҫԭ�ϡ�ʵ����ͨ����������װ����ȡ���������������
Ҫ��ش�
Ҫ��ش�
��ʵ����ʹ��ϡ����ͽ���п��ȡ�����Ļ�ѧ��Ӧ����ʽΪ__________________�����ַ����Ƶõ�����������������________________(ԭ����__________________________)��ˮ�������ɽ���������ͨ��װ��_________________��ȥ��
��ʵ���пɲ���__________װ���ռ�����������������ļ���ƿ��װ��G��ʽ���ñ��ã�ԭ����__________________________________��
(2)(5��)ijУ�����о�С�鵽��Ƴ����죬�˽������ͭ����Ҫ�����������£�
(2)(5��)ijУ�����о�С�鵽��Ƴ����죬�˽������ͭ����Ҫ�����������£�
����������ṩ����Ϣ�ش�
�����̢��ˮ�к��е�������_________________________________��
�ھ��ⶨ�����̢��з�ˮ��pHΪ4����ˮ��һ��û��________________________��
�����̢���ˮ�г����̢�����������⣬������_______________________��
����Ƽ�����֤������ƶϣ�
______________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ�ɽ������������ѧ ���ͣ��ƶ���
(1)(6��)��������������壬������ˮ��������Ϊδ����������Դ���Ǻ��칤ҵ�ĸ���ȼ�ϣ�Ҳ�ǻ�����������Ҫԭ�ϡ�ʵ����ͨ����������װ����ȡ���������������
Ҫ��ش�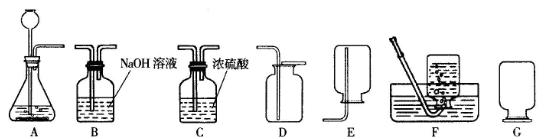
Ҫ��ش�
��ʵ����ʹ��ϡ����ͽ���п��ȡ�����Ļ�ѧ��Ӧ����ʽΪ__________________�����ַ����Ƶõ�����������������________________(ԭ����__________________________)��ˮ�������ɽ���������ͨ��װ��_________________��ȥ��
��ʵ���пɲ���__________װ���ռ�����������������ļ���ƿ��װ��G��ʽ���ñ��ã�ԭ����__________________________________��
(2)(5��)ijУ�����о�С�鵽��Ƴ����죬�˽������ͭ����Ҫ�����������£�
(2)(5��)ijУ�����о�С�鵽��Ƴ����죬�˽������ͭ����Ҫ�����������£�
����������ṩ����Ϣ�ش�
�����̢��ˮ�к��е�������_________________________________��
�ھ��ⶨ�����̢��з�ˮ��pHΪ4����ˮ��һ��û��________________________��
�����̢���ˮ�г����̢�����������⣬������_______________________��
����Ƽ�����֤������ƶϣ�
______________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ�ɽ������������ѧ ���ͣ���Ϣ������
(1)(6��)��������������壬������ˮ��������Ϊδ����������Դ���Ǻ��칤ҵ�ĸ���ȼ�ϣ�Ҳ�ǻ�����������Ҫԭ�ϡ�ʵ����ͨ����������װ����ȡ���������������
Ҫ��ش�

Ҫ��ش�
��ʵ����ʹ��ϡ����ͽ���п��ȡ�����Ļ�ѧ��Ӧ����ʽΪ__________________�����ַ����Ƶõ�����������������________________(ԭ����__________________________)��ˮ�������ɽ���������ͨ��װ��_________________��ȥ��
��ʵ���пɲ���__________װ���ռ�����������������ļ���ƿ��װ��G��ʽ���ñ��ã�ԭ����__________________________________��
(2)(5��)ijУ�����о�С�鵽��Ƴ����죬�˽������ͭ����Ҫ�����������£�

(2)(5��)ijУ�����о�С�鵽��Ƴ����죬�˽������ͭ����Ҫ�����������£�
����������ṩ����Ϣ�ش�
�����̢��ˮ�к��е�������_________________________________��
�ھ��ⶨ�����̢��з�ˮ��pHΪ4����ˮ��һ��û��________________________��
�����̢���ˮ�г����̢�����������⣬������_______________________��
����Ƽ�����֤������ƶϣ�
______________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com