
����Ŀ����ʯͷֽ��������ĥ�ɷ�ĩ��ʯͷΪ��Ҫԭ�����ɵġ�����ֽ��ˮ��̲���ȼ�գ�����Ҫ���Dz��ÿ�����ֽ���dz��������ճ̱�����ǩֽ�ȶ���������̼���Ϊ��Ҫԭ�ϵĵ�̼��ʯͷֽ����Ϊ�ⶨ����̼��Ƶĺ���������С���ͬѧ��ȡ50g��ֽ��Ʒ���ֱ���5ֻ�ձ��н�����ʵ�飬ʵ�����ݼ��±�������ֽ�������ɷּȲ�����ˮ��Ҳ����ˮ�����ᷴӦ����
�ձ��� | �ձ��� | �ձ��� | �ձ��� | �ձ��� | |
������Ʒ������/g | 10 | 10 | 10 | 10 | 10 |
����ϡ���������/g | 10 | 20 | 30 | 40 | 50 |
��ַ�Ӧ���������������/g | 0.88 | 1.76 | X | 3.52 | 3.52 |
��1������X��ֵΪ________��
��2�����ձ�_______��̼�����ȫ��Ӧ��
��3������Ʒ��̼��Ƶ���������_____��
���𰸡�2.64 �ܢ� 80%
��������
��1������������ϵ�����ж�x��ֵ��
��2���ݱ����ṩ�����ݿ�֪�����ձ��м���ϡ����������ֱ���10g��20g��30g��40gʱ�����ɶ�����̼�����������ӣ��ձ���ϡ����ÿ����10g�����ɵ����������0.88g�����ձ����е���������Ҳ��3.52g����˵���ձ�������Ʒ��ϡ����ǡ����ȫ��Ӧ���ձ����е�������ʣ�ࣻ
��3�����ݲ���������̼���������Լ���̼��Ƶ���������һ�����Լ�����Ʒ��̼��Ƶ�����������
�⣺��1���ɷ�Ӧ�����ݿ�֪����10g��Ʒ�н���30g����ʱ��̼�����ȫ��Ӧ�����ɶ�����̼������Ϊ��0.88g��3=2.64g��
��2���ݱ����ṩ�����ݿ�֪�����ձ��м���ϡ����������ֱ���10g��20g��30g��40gʱ�����ɶ�����̼�����������ӣ��ձ���ϡ����ÿ����10g�����ɵ����������0.88g�����ձ����е���������Ҳ��3.52g����˵���ձ�����̼��ƺ�ϡ����ǡ����ȫ��Ӧ���ձ����е�������ʣ����̼�����ȫ��Ӧ��
��3���ɱ������ݿ�֪��10g��Ʒ�е�̼�����ȫ��Ӧʱ�����ɶ�����̼��������3.52g����10g��Ʒ�е�̼��Ƶ�����Ϊx����

![]()
x=8g��
��Ʒ��̼��Ƶ�����������![]() ��100%=80%��
��100%=80%��
����Ʒ��̼��Ƶ���������Ϊ80%��


 ������ϵ�д�
������ϵ�д� �żӾ���ϵ�д�
�żӾ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ1�Dzⶨ����������������ʵ��װ�ã������ʵ��ش����⡣
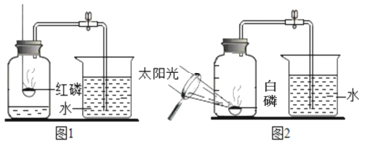
��1��ʵ��ó��Ľ�����____________��
��2��ʵ����Ϻ������뼯��ƿ��ˮ���������ԭ���������1/5������Ϊ������һ�����ԭ�������:_____________��д�����㼴�ɣ�
��3��ȼ�ս�������ƿ��ʣ���������Ҫ��________���ɱ�ʵ�����֪�����������������_______________����ѧ������____________��
��4������������ľ̿�ۣ���ʵ���ܷ��óɹ���__________����ܡ����ܡ����������ԭ��________________��
��5��ijͬѧ��ʵ����з�˼������˸Ľ���������ͼ2��ʾ��������Ϊ�Ľ�����ŵ��ǣ�____________(д�����㼴��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��д�����з�Ӧ�����ֻ���ű���ʽ��
��1��������������ȼ��____________________________________________��
��2��ʵ������ȡ������̼��_______________________ ��
��3������������ȼ�գ�_________________��
��4��þ���ڿ�����ȼ�գ�___________________ ��
��5������˫��ˮ��ȡ������_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���Ұ���ͼ��ʾװ����ȡ������̼������֤������̼�����ʣ��Իش��������⡣
��1��ʵ������ȡ������̼��ԭ�����û�ѧ����ʽ��ʾ��_______________��
��2����a��b�ĵ��ܿ�����ʱ���ɼ������ɵĶ�����̼���� ������B�п�����ʵ��������_________����ѧ��Ӧ����ʽΪ_____________��
��3������֤������̼����ˮ��Ӧ����C��Ӧװ�Լ���_________��
��4����a��d�ĵ��ܿ�����ʱ���۲쵽D��ȼ�ŵ������ɵ͵�����Ϩ��˵��������̼���е�����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
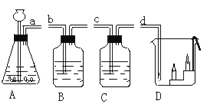
����Ŀ������A-D�dz��л�ѧ�е��ĸ�ʵ�飬�밴Ҫ����գ�

��1��д��C�з�Ӧ�Ļ�ѧ����ʽ____________��
��2��Dʵ�����ձ��۵�������_____________��
��3��Bʵ��ɹ��Ĺؼ���__________������ţ���
��װ�������Ժã���ʵ��ǰ�н�ֹˮ�У��ۺ�������������
����ȴ���ٴ�ֹˮ�У���Ҫѡ�ý����ڵĿ�����
��4������A-D�ĸ�ʵ�����ܴﵽʵ��Ŀ������ȷ����___________������ĸ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I����һ��������п��Ͷ�뵽��������������ͭ�Ļ����ҺA�У���ֽ��裬���ˡ��ش��������⡣
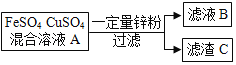
��1����������C�м�������ϡ���ᣬ���������ɡ�����C��һ����__����ҺB�г�ˮ�⣬һ����__��
��2��������ҺA�м��������п�ۣ�д��������C�м����������ᣬ�����ķ�Ӧ�Ļ�ѧ����ʽΪ__________��_______��
II��Ϊ����֤�������ǿ������֤�����غ㶨�ɣ�ͬѧ���������ͼ��ʾ��ʵ�顣

��1������ʵ��һ��ʵ�����������֤ͭ��п�������ǿ����ͬʱ����֤�����غ㶨�ɣ�����ҺA������__������ţ���
a��ϡ���� b����������Һ c���Ȼ�������Һ d������п��Һ e������ͭ��Һ
��2�����������Լ���Ʒ�Ӧ����֤ͭ�����Ľ�����ԣ���ѧ��Ӧ����ʽΪ____������Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���⻯þ����ѧʽΪMgH2����һ�ֳ��õ�������ij��ȤС����ѡ������ͼװ���Ʊ��⻯þ��
���������ϣ�
��1���⻯þ��������������þ�ۼ����Ƶá��⻯þ��ˮ��Ӧ����������þ[Mg(OH)2]�����������ų�������

��2��������þ����ˮ��Ӧ����״�����������ܶ�Ϊ0.09g/L��
��ʵ�鲽�裩
��������װ�ã��١�����
��װ��ҩƷ����Һ©��������װ����ͨ��������D���ռ��������鴿��Cװ�ü��ȡ�����ͨ��ƽ�ȵ���������
��ʵ�����ʱ����ֹͣ���ȣ���װ����ȴ������ֹͣͨ��������
�ش��������⣺
��1���뽫������еIJ�����������____��
��2��װ��A�з�Ӧ�Ļ�ѧ����ʽ____��A���÷�Һ©������ϡ������ŵ���____���Ƶõ��⻯þ����____���档
��3��ʵ�鿪ʼʱҪ����ͨ��������Ŀ�ģ�����ֹ���������ȵĿ�������ը�⣬����____��Bװ�õ�������____��
��4��ʵ�����ͬѧ��������ͼװ�ü��õ����⻯þ�Ĵ��ȡ�ȡһ��������Ʒ����ͼ����Y�ι�һ�ˣ�ʵ�鿪ʼ��Y�ι���б��ˮ����Ʒ�Ӵ������ռ�������178ml������Ʒ���⻯þ��������____g��
��5����������Ľ��ƫ���ܵ�ԭ����____��
A��Y�ι�����ˮ�����Ҳ��ų���������
B������������Ͳ̫����δ�������ó��Ͷ���
C��δ��ȴ�����¾Ͷ���
D��ʵ��ǰY�ι����п���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
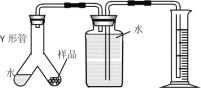
����Ŀ����������װ�ý����������ȡ���ռ����ش��������⣺
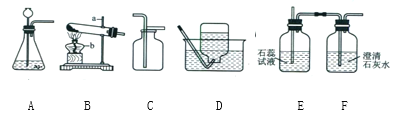
��1����д���������ƣ�a._________��
��2��ʵ�����ﲻʹ�ô�����ȡ����ʱ����ѡ�õķ���װ����______������ĸ����ʵ������ȡCO2����ʱ������Ӧ�Ļ�ѧ����ʽΪ___________���ռ�CO2һ�㲻ѡ��װ��D��ԭ����__________��ͬѧ��ͼ��װ��A��E��F����Ʊ�CO2���岢��֤������ʣ���װ��AӦ��������װ��______��ѡ�E����F ������Eװ���е�������_________��װ��E��F�зֱ�����Ӧ�ķ���ʽ___________��_________��
��3����װ��A�з�Ӧ���ھ��ң��Ӱ�ȫ�Ƕȿ��ǿɲ�ȡ�Ĵ�ʩ��________������ţ���
a�����ݻ���С����ƿ
b��������©��������Ͳ����Һ��ĵμ��ٶ�
c�����ȷ�Ӧ��
d������Һ�巴Ӧ���Ũ��
��4��ij��ѧ��ȤС������ʦָ������ȡ������ռ���������̽��������һЩ���ʡ�
���������ϣ�
a������ͨ����������д̼�����ζ����ɫ���壬�ܶȱȿ���С����������ˮ��
b����ˮ�ǰ�����NH3����ˮ��Һ�����м�����ʡ�
c������Һ��ʹ��ɫ��̪��Һ��졣
��ͼΪ�ϳɰ���ģ��ʵ��װ��ͼ���������������ۺ�ʯ������ɵĻ�����
���裺�ټ�������ԣ���ʹп����ϡ���ᷴӦһ��ʱ�䣻����Y��ʢ���������ƺ��Ȼ�隣�����Һ��һ���ȣ�NH4Cl + NaNO2 ![]() NaCl + 2H2O + N2�� �����ܵ����������Ͽ�ʱ���ٵ�ȼ�������·��ľƾ��ƽ��м��ȡ�
NaCl + 2H2O + N2�� �����ܵ����������Ͽ�ʱ���ٵ�ȼ�������·��ľƾ��ƽ��м��ȡ�
�ٷ�Ӧ��ʼǰʹп����ϡ���ᷴӦһ��ʱ���Ŀ���ǣ�__________��
�ڵ���ƿ�г���__________ ����,��ʾ�а������ɡ�
��д�������������ɰ����Ļ�ѧ����ʽ_______________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������άϵ��Ҫˮ��������ճ������ũҵ�����벻��ˮ��
��1�����С�ˮ���У����ڴ��������___(�����)��
a����ˮ b����ˮ c������ˮ d����ˮ
��2��ˮ��ͨ�������·ֽ�Ļ�ѧ����ʽΪ_____________��
��3������ˮ��ͨ��ɳ�dz�ȥˮ�������Թ������ʡ��豭�г����IJ�����������������õ�___ԭ������ȥ��ˮ��Ư���IJ�Ҷ�����Dz����ø÷�����Ӳˮ�����������п���____�ķ�������ˮ��Ӳ�ȡ�
��4��ˮ�л��̲��ŷḻ�Ķ�����Դ��������Դ�Ϳ�����Դ�ȡ������� 2017��05��18�� 11:23�����ҹ��Ϻ�ȫ���״��Կ��ɿ�ȼ���ɹ����ⲻ�������ҹ���Ȼ��ˮ�����Ϳ����ĺ��ļ����õ���֤��Ҳ��־���й�����һ������ۺ�ʵ���ﵽ���綥��ˮƽ��

�ٿ�ȼ�������ɼ��飨CH4����ˮ�ڸ�ѹ���µ��������γɵ����״�Ľᾧ�����Ԥ�⺣ˮ�п�ȼ����Դ�����൱���ѷ��ֵ�_______��______����Ȼ��������ʯȼ�ϵ��������ϣ������繫�ϵ�һ������Ч��δ�������Դ����ȼ����Ҫ�ɷּ�����ȫȼ�����ɶ�����̼��ˮ����д������ȼ�յĻ�ѧ����ʽ��_________��
���Ҵ�Ҳ��һ�������Դ����ȫȼ��Ҳ���ɶ�����̼��ˮ�����Ҵ�һ�����е�Ԫ����______��
�����ȼ����������ں��ף����Կ����Ѷ�ʮ�־�������ɲ������¼�������Ĵ���й©������������ǿ�ҵ�����ЧӦ��������������ЧӦ������Σ������______����ѡ��
A�������ϵIJ��溦����; B����ƽ������; C������,����籩����; D�����ظɺ�,ɳĮ���������; E��������ʧȥ��Ϣ�ء�
��5��Ŀǰ����������ЧӦ��������Ҫ�Ƕ�����̼��ijУѧ���ڡ������ж�����̼����Ĺ�������ı��ۻ��ϣ������¹۵㣺
�۵�һ��������̼�����������ЧӦ��ʹ�����쳣����������������ѣ�
�۵����������̼����Ϊֲ��______�����ṩ���㹻��ԭ�ϣ��ǽ�����ũ������յ�ԭ��֮һ��
��д���ճ�����ȼ��ú̿��ľ̿�Ĺ����У��ж�����̼���ɵ�һ����ѧ����ʽ______��
�����˽�����Խ������еĶ�����̼���͵������������ء� ���п�ѧ�ҵ��������������Ӻ�ˮ����ȣ����º�������������������̼ʹ��ˮ������ӵ�ԭ����______���û�ѧ����ʽ��ʾ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com