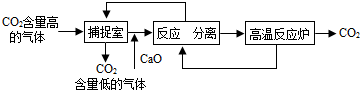
��2012?�ֿ�����ģ������̼���С�������ᴩ���Ϻ������Ľ�����
��1������̼���С��Ľ�������˶�����̼������ŷţ��ܼ���
����ЧӦ
����ЧӦ
�ij̶ȣ���Ȼ�������Ķ�����̼����Ҫ;����
�������
�������
������д��һ���ճ������з��ϡ���̼���á������������
���ֹصƣ���������
���ֹصƣ���������
��
��2��������ѧ��PaulSabatier���á����ת��������ʹCO
2��H
2�ڴ�������������CH
4��H
2O����д���÷�Ӧ�Ļ�ѧ����ʽ
��
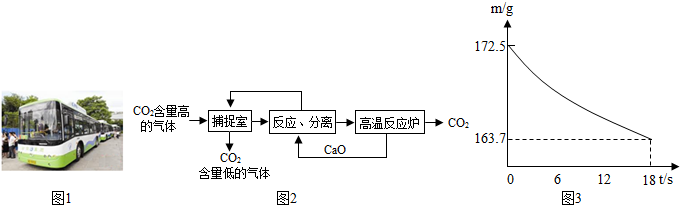
��3�������п�ѧ��������á�̼��������������ҵ�����ж�����̼���ŷ�������̼����������ָͨ��һ���ķ���������ҵ�����в�����CO
2����������д�������ã�������������NaOH��Һ��������CO
2��������ͼ��ʾ����������������δ��������ٲ����з�����Ӧ�Ļ�ѧ����ʽΪ��
?CO2+2NaOH�TNa2CO3+H2O
?CO2+2NaOH�TNa2CO3+H2O
��
�ڰ�CaO���뷴Ӧ����������H
2O��Ӧ����д���÷�Ӧ�Ļ�ѧ����ʽ��
CaO+H2O�TCa��OH��2
CaO+H2O�TCa��OH��2
�����ô˷�Ӧ�������ƿ�����ʳƷ
����
����
����
�ۡ���Ӧ���롱�У��õ��������ʵĻ���������
����
����
���ù�����̼��ƣ�
�����������У�����ѭ�����õ�������
CaO��NaOH
CaO��NaOH
��
��4��ȡ10g̼��ƹ�����¼��ȣ�һ��ʱ���ֹͣ���ȣ����ʣ������и�Ԫ�ص���������Ϊ50%���������ж���ȷ����
A
A
��
A��������2g������̼
B��ʣ���������Ϊ5g
C��������5.6g������
D��ʣ��̼��Ƶ�����Ϊ8g��




 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�