
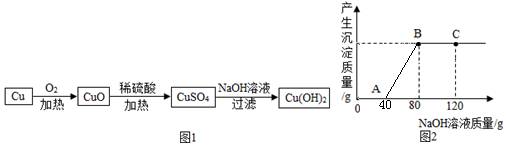
·ÖĪö øł¾ŻĶŹĒŗģÉ«”¢Ńõ»ÆĶŹĒŗŚÉ«½ųŠŠ·ÖĪö½ā“š£»øł¾ŻĒāŃõ»ÆÄĘÓėĮņĖįŗĶĮņĖįĶµÄ»ģŗĻČÜŅŗµÄ·“Ó¦½ųŠŠ·ÖĪö½ā“š£¬øł¾Ż×ų±źÖŠĢį¹©µÄŹż¾ŻŅŌ¼°»Æѧ·½³ĢŹ½½ųŠŠ·ÖĪö½ā“š¼“æÉ£®
½ā“š ½ā£ŗ£Ø1£©ĶŹĒŗģÉ«µÄ£¬ÓėŃõĘų·“Ӧɜ³ÉµÄŃõ»ÆĶŹĒŗŚÉ«µÄ£¬¹Ź»į¹Ū²ģµ½ŗģÉ«¹ĢĢå±ä³ÉŗŚÉ«µÄĻÖĻ󣬹ŹĢī£ŗŗģÉ«¹ĢĢå±äŗŚ£»
£Ø2£©Ńõ»ÆĶÄÜÓėĮņĖį·“Ӧɜ³ÉĮņĖįĶ£¬Č”Ņ»¶ØĮæµÄCuO·ÅČėŹ¢ÓŠŅ»¶ØÖŹĮæ19.6%Ļ”ĮņĖįČÜŅŗµÄÉÕ±ÖŠ³ä·Ö·“Ó¦ŗó£¬ĻņÉÕ±ÖŠÖšµĪ¼ÓČė10%µÄNaOHČÜŅŗ£¬øł¾ŻĶ¼ĻóæÉŅŌ擳ö£¬æŖŹ¼Ć»ÓŠ³ĮµķÉś³É£¬¹ŹæŖŹ¼ŹĒĒāŃõ»ÆÄĘÓėŹ£ÓąµÄĮņĖį·“Ó¦£¬“ļµ½AµćŹ±ĮņĖįĒ”ŗĆĶźČ«·“Ó¦£¬“ĖŹ±µÄČÜÖŹŹĒĮņĖįĶŗĶĮņĖįÄĘ£¬µ½“ļCµćŹ±£¬ĒāŃõ»ÆÄĘŹ£Óą£¬“ĖŹ±µÄČÜÖŹŹĒĮņĖįÄĘŗĶŹ£ÓąµÄĒāŃõ»ÆÄĘ£¬¹Ź¶ąµÄČÜÖŹŹĒĒāŃõ»ÆÄĘ£¬¹ŹĢī£ŗNaOH£»
£Ø3£©øł¾ŻĶ¼Ź¾æÉŅŌ擳ö£¬ÓėĮņĖįĶ·“Ó¦µÄĒāŃõ»ÆÄĘČÜŅŗµÄÖŹĮæĪŖ40g£¬¹ŹĒāŃõ»ÆÄʵÄÖŹĮæĪŖ£ŗ40g”Į10%=4g£¬ÉčÉś³ÉµÄĒāŃõ»ÆĶµÄÖŹĮæĪŖx
2NaOH+CuSO4ØTCu£ØOH£©2”ż+Na2SO4
80 98
4g x
$\frac{80}{98}=\frac{4g}{x}$
x=4.9g
£Ø4£©øł¾Ż·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗH2SO4+2NaOHØTNa2SO4+2H2O£¬2NaOH+CuSO4ØTCu£ØOH£©2”ż+Na2SO4£¬¹ŹæÉŅŌµĆ³ö£ŗ2NaOH--Na2SO4£¬
ĻūŗĵÄĒāŃõ»ÆÄʵÄ×ÜÖŹĮæĪŖ£ŗ80g”Į10%=8g
ÉčÉś³ÉµÄĮņĖįÄʵÄÖŹĮæĪŖy
2NaOH--Na2SO4£¬
80 142
8g y
$\frac{80}{142}=\frac{8g}{y}$
y=14.2g
£Ø5£©CuO+H2SO4ØTCuSO4+H2O£¬2NaOH+CuSO4ØTCu£ØOH£©2”ż+Na2SO4£¬H2SO4+2NaOHØTNa2SO4+2H2O£¬æÉŅŌµĆ³öH2SO4--2NaOH
ÉčĮņĖįµÄÖŹĮæĪŖz
H2SO4--2NaOH
98 80
z 8g
$\frac{98}{80}=\frac{z}{8g}$
z=9.8g
¹ŹĻ”ĮņĖįµÄÖŹĮæĪŖ£ŗ$\frac{9.8g}{19.6%}$=50g
“š£ŗ£Ø3£©ĖłµĆCu£ØOH£©2³ĮµķµÄÖŹĮæŹĒ4.9g£»
£Ø4£©BµćŹ±£¬ČÜŅŗÖŠČÜÖŹµÄÖŹĮæŹĒ14.2g£»
£Ø5£©ŹµŃéĖłÓƵÄĻ”ĮņĖįÖŹĮæŹĒ50g£®
µćĘĄ ±¾Ģāæ¼²éµÄŹĒøł¾Ż»Æѧ·½³ĢŹ½µÄ¼ĘĖćµÄÖŖŹ¶£¬Ķź³É“ĖĢā£¬æÉŅŌŅĄ¾ŻŅŃÓŠµÄÖŖŹ¶½ųŠŠ£®


 ŠĀĖ¼Ī¬¼ŁĘŚ×÷Ņµŗ®¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø
ŠĀĖ¼Ī¬¼ŁĘŚ×÷Ņµŗ®¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ĪŖĮĖ²ā¶ØijŹÆ»ŅŹÆÖŠĢ¼ĖįøʵÄÖŹĮæ·ÖŹż£¬æĪĶāŠĖȤŠ”×é½ųŠŠĮĖČēĻĀŹµŃé£ŗ³ĘČ”10æĖŹÆ»ŅŹÆ·ÅŌŚÉÕ±Ąļ£¬ĶłÉÕ±ÖŠ¼ÓČė×ćĮæµÄČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄĻ”ŃĪĖįČÜŅŗ£®·“Ó¦¹ż³ĢÖŠÉÕ±ÄŚĪļÖŹ×ÜÖŹĮæµÄ±ä»ÆČēĶ¼ĖłŹ¾£®
ĪŖĮĖ²ā¶ØijŹÆ»ŅŹÆÖŠĢ¼ĖįøʵÄÖŹĮæ·ÖŹż£¬æĪĶāŠĖȤŠ”×é½ųŠŠĮĖČēĻĀŹµŃé£ŗ³ĘČ”10æĖŹÆ»ŅŹÆ·ÅŌŚÉÕ±Ąļ£¬ĶłÉÕ±ÖŠ¼ÓČė×ćĮæµÄČÜÖŹÖŹĮæ·ÖŹżĪŖ10%µÄĻ”ŃĪĖįČÜŅŗ£®·“Ó¦¹ż³ĢÖŠÉÕ±ÄŚĪļÖŹ×ÜÖŹĮæµÄ±ä»ÆČēĶ¼ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 ŌŚĶŠÅĢĢģĘ½µÄĮ½±ßø÷·ÅŅ»Ö»µČÖŹĮæµÄÉÕ±£¬ĻņĮ½Ö»ÉÕ±ÖŠ·Ö±š×¢ČėĻąĶ¬ÖŹĮ攢ĻąĶ¬ÖŹĮæ·ÖŹżµÄĻ”ĮņĖį£¬ĢģĘ½Ę½ŗā£®ČōĻņ×ó±ßÉÕ±ÖŠĶ¶ČėÉŁĮæµÄŠæĮ££¬ĻņÓŅ±ßÉÕ±ÖŠĶ¶ČėµČÖŹĮæµÄĆ¾Ģõ£ØČēĶ¼ĖłŹ¾£©£¬“ż³ä·Ö·“Ó¦ŗ󣬷¢ĻÖŠæĮ£ŗĶĆ¾Ģõ¾łĪŽŹ£Óą£¬ŌņĢģĘ½ÖøÕė£Ø””””£©
ŌŚĶŠÅĢĢģĘ½µÄĮ½±ßø÷·ÅŅ»Ö»µČÖŹĮæµÄÉÕ±£¬ĻņĮ½Ö»ÉÕ±ÖŠ·Ö±š×¢ČėĻąĶ¬ÖŹĮ攢ĻąĶ¬ÖŹĮæ·ÖŹżµÄĻ”ĮņĖį£¬ĢģĘ½Ę½ŗā£®ČōĻņ×ó±ßÉÕ±ÖŠĶ¶ČėÉŁĮæµÄŠæĮ££¬ĻņÓŅ±ßÉÕ±ÖŠĶ¶ČėµČÖŹĮæµÄĆ¾Ģõ£ØČēĶ¼ĖłŹ¾£©£¬“ż³ä·Ö·“Ó¦ŗ󣬷¢ĻÖŠæĮ£ŗĶĆ¾Ģõ¾łĪŽŹ£Óą£¬ŌņĢģĘ½ÖøÕė£Ø””””£©| A£® | ĻČĻņÓŅĘ«£¬ŗóĻņ×óĘ« | B£® | ĻČĻņ×óĘ«£¬ŗóĻņÓŅĘ« | ||
| C£® | Ņ»Ö±Ļņ×óĘ« | D£® | Ņ»Ö±ĻņÓŅĘ« |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
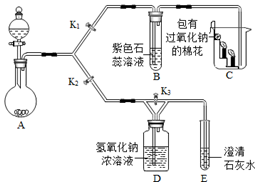
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
 ĒāŃõ»ÆøĘ³¤ĘŚ“ę·ÅČŻŅ×±äÖŹ£®Ä³Š”×éŌŚŹµŃ鏱ȔĮĖŅ»Ęæ“ę·Å¶ąÄźµÄĒāŃõ»ÆøĘĄ“¼ģ²é±äÖŹĒéæö£®Č”12.2gѳʷ£¬¼ÓČė32.8gĖ®£¬ŠĪ³ÉŠü×ĒŅŗ£¬Č»ŗóÖš½„µĪ¼Ó29.2%µÄŃĪĖį³ä·Ö·“Ó¦£®¼ÓČėŃĪĖįµÄÖŹĮæÓė׶ŠĪĘæÖŠĪļÖŹµÄÖŹĮæ¹ŲĻµČēĶ¼£®
ĒāŃõ»ÆøĘ³¤ĘŚ“ę·ÅČŻŅ×±äÖŹ£®Ä³Š”×éŌŚŹµŃ鏱ȔĮĖŅ»Ęæ“ę·Å¶ąÄźµÄĒāŃõ»ÆøĘĄ“¼ģ²é±äÖŹĒéæö£®Č”12.2gѳʷ£¬¼ÓČė32.8gĖ®£¬ŠĪ³ÉŠü×ĒŅŗ£¬Č»ŗóÖš½„µĪ¼Ó29.2%µÄŃĪĖį³ä·Ö·“Ó¦£®¼ÓČėŃĪĖįµÄÖŹĮæÓė׶ŠĪĘæÖŠĪļÖŹµÄÖŹĮæ¹ŲĻµČēĶ¼£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
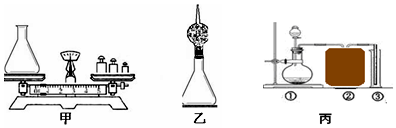
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ĪļÖŹ | Ėłŗ¬ŌÓÖŹ | ³żČ„ŌÓÖŹµÄ·½·Ø | |
| A | O2 | CO | µćČ¼»ģŗĻĘųĢå |
| B | NaOH | NaCl | ŹŹĮæµÄĻ”ŃĪĖįČÜŅŗ |
| C | CuO | Cu | ŌŚ“æĒāĘųĮ÷ÖŠ¼ÓČČ |
| D | H2 | Ė®ÕōĘū | ĶعżŹ¢ÓŠÅØĮņĖįµÄĻ“ĘųĘæ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com