
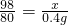
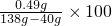 %=0.5%
%=0.5%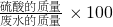 %�������ˮ�����������������
%�������ˮ�����������������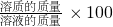 %�����ʽҪ������ã����ʵ���������Һ�����������ʵ�������������������ֻҪ֪�������������������������һ������
%�����ʽҪ������ã����ʵ���������Һ�����������ʵ�������������������ֻҪ֪�������������������������һ������ 


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�γ��ж�̨��������꼶��ѧ�ڵ�һ���¿���ѧ�Ծ����������� ���ͣ������
ijУ�����ꡱ����С���ͬѧ��ȡ�ս������ˮ��ˮ������pH̽ͷ����pH��������ÿ�����β�һ��pH�������ݼ��±���
| �ⶨʱ�� | 3:10 | 3:15 | 3:20 | 3:25 | 3:30 | 3:35 | 3:40 |
| pH | 4.95 | 4.94 | 4.93 | 4.88 | 4.86 | 4.85 | 4.84 |
| �Լ� | ϡ���� | ʳ��ˮ | ����ˮ | ��ľ��ˮ | ʯ��ˮ |
| ��ɫ | �� | �� | �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�γ��ж�̨��������꼶��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
ijУ�����ꡱ����С���ͬѧ��ȡ�ս������ˮ��ˮ������pH̽ͷ����pH��������ÿ�����β�һ��pH�������ݼ��±���
|
�ⶨʱ�� |
3:10 |
3:15 |
3:20 |
3:25 |
3:30 |
3:35 |
3:40 |
|
pH |
4.95 |
4.94 |
4.93 |
4.88 |
4.86 |
4.85 |
4.84 |
��1��������ѧ֪ʶ���Ʋ⡰������ˮ����pH 7�����������������������������pH��ԭ���� ��
��2�������������ݣ��ж�������ˮ�Ƿ�Ϊ�����ꡱ�� ��
��3�������飬��һ������һ�����᳧�����������в���SO2����һ����Ƴ�����Щ��ʹ�õ�ȼ����Ҫ��ú���Է��������һ���������ꡱ����Ҫԭ���� ������Ϊ���Բ�ȡ��Щ��Ч��ʩ������һ�����ġ����ꡱ�������2���� �� ��
��4��ijͬѧ���Ƴ�һ�ֻ�ɫ�Ļ�֭�����뵽��ͬ�Լ��У��۲쵽���������£�
|
�Լ� |
ϡ���� |
ʳ��ˮ |
����ˮ |
��ľ��ˮ |
ʯ��ˮ |
|
��ɫ |
�� |
�� |
�� |
�� |
�� |
Сǿ���ݱ�����Ϣ��������ʵ�飺
�øû�֭ȥ���鸽��С�������ŷŷ�ˮ������ԣ��Ժ�ɫ����˷�ˮ�� �ԡ�
b.�øû�֭��֪���л������������ԣ���������ϱ��е� ��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com