
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ȡ������Ʒ���Թ��У��μ�ϡ���ᣮ | ��������ð�� | ��Ʒ�������к���CaCO3�� |
| �ڽ�����������ͨ�����ʯ��ˮ�� | ��ʯ��ˮ����� |
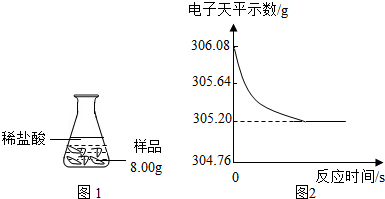
���� ʵ��̽��һ
[���ʵ��]����̼������ӵļ��鷽���������ʵ�飮
ʵ��̽������
[���ݴ���]��������������Һ�����ն�����̼������������㣮
[�����뷴˼]
��1������̼���������ķ�Ӧ��д����ƿ������������Ļ�ѧ����ʽ��
��2�����������Ҫ��������������CaCO3������������ƫС��
��3������ϡ��������ʣ����м�⣮
��4������������Ļӷ��ԣ�
��� �⣺ʵ��̽��һ
[���ʵ��]����̼��������ᷴӦ�����˶�����̼���壬������̼��ʹ�����ʯ��ˮ����ǣ����ԣ����ʵ�����£�
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ȡ������Ʒ���Թ��У��μ� ϡ���� | �������ݲ��� | ��������Ʒ�к���CaCO3�� |
| �ڽ�����������ͨ�����ʯ��ˮ | �ڳ���ʯ��ˮ����� |
���� ʵ���ǻ�ѧ����Ҫ��ɲ��֣���ȷ��ʵ������ǵó���ѧ���۵�ǰ������֮һ�����Ҫѧ�����ʵ�顢����ʵ�顢����ʵ�飬Ϊ�ó���ȷ�Ľ��۵춨������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷���ˮ����Ӳˮ����ˮ | |
| B�� | ��ˮ��������粒�����������ƹ��� | |
| C�� | ��ϡ����������������Ƿ���� | |
| D�� | �÷�̪��Һ����ϡ�����ʳ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��Ԫ��������Ĺ�ϵ���У�
��Ԫ��������Ĺ�ϵ���У��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������� | |
| B�� | ̼���⡢��Ԫ�ص�������Ϊ6��1��8 | |
| C�� | ���������������� | |
| D�� | ���������̼���⡢��ԭ�ӵĸ�����Ϊ1��2��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �٢ڢ� | C�� | �ڢܢ� | D�� | �ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��ͭ��п������ĩ�м������� | |
| B�� |  ��ͭƬ���뵽һ��������������Һ�� | |
| C�� |  ��������ȫ��ͬ��ϡ�����зֱ����п�ۡ�þ�� | |
| D�� |  ��������ȫ��ͬ��ϡ�����зֱ����п�ۡ�þ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | �������� | �����Լ� | |
| A | ���� | ͭ�� | ϡ���� |
| B | NaCl | Na2CO3 | ϡH2SO4 |
| C | CO2 | CO | ���ȵ�CuO |
| D | KCl | KNO3 | H2O |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com