
| ��� | ����ϡ���������/�� | ʣ����������/�� |
| ��һ�� | 20 | 11 |
| �ڶ��� | 20 | 6 |
| ������ | 20 | 2.8 |
| ���Ĵ� | 20 | n |
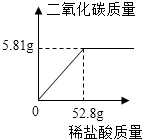
���� ��1������ͼ�����ݿ�֪����1��2�η�Ӧ��������ʼ��ٵ�������Ϊ5g������3�η�Ӧ��������ʼ��ٵ�����Ϊ3.2g��˵����ʱ̼����ѷ�Ӧ�꣬���ٲ������壬�ʱ���n����ֵΪ2.8��
��2������ͼ�����ݿ�֪����ȫ��Ӧ��ʣ��������ʵ�����Ϊ2.8g��ʯ��ʯ��Ʒ��������ȥʣ��������ʵ�����������Ʒ��̼��Ƶ�������Ȼ���������������ʽ���㼴�ɣ�
��4������ͼ�����ݿ�֪����1��2�η�Ӧ��������ʼ��ٵ�������Ϊ5g��˵��20gϡ����ǡ������ʯ��ʯ�е�5g̼�����ȫ��Ӧ������̼��������ᷴӦ�Ļ�ѧ����ʽ�͵�1����ȫ��Ӧ��̼��Ƶ����������ɼ������һ�β��뷴Ӧ��HCl������Ȼ��������ʵ�����������ʽ���㼴�ɣ�
��3�����ݼ������̼����������������������������μӷ�Ӧ��ϡ����������Ͷ�����̼��������Ȼ�������ͼ��
��� �⣺��1������ͼ�����ݿ�֪����1��2�η�Ӧ��������ʼ��ٵ�������Ϊ5g������3�η�Ӧ��������ʼ��ٵ�����Ϊ3.2g��˵����ʱ̼����ѷ�Ӧ�꣬���ٲ������壬���Ա���n����ֵΪ2.8��
��2����Ʒ��̼��Ƶ���������Ϊ��$\frac{16g-2.8g}{16g}$��100%=82.5%��
����Ʒ��̼��Ƶ���������Ϊ82.5%��
��4�����������20gϡ����ǡ������ʯ��ʯ�е�5g̼�����ȫ��Ӧ��
���һ�β��뷴Ӧ��HCl����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73
5g x
$\frac{100}{5g}$=$\frac{73}{x}$
x=3.65g��
���������ʵ���������Ϊ��$\frac{3.65g}{20g}$��100%=18.25%��
��4��ͨ��������֪̼�����Ʒ�к���̼�������Ϊ13.2g��
����Ҫ��ϡ��������Ϊy�����ɵĶ�����̼����Ϊz��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
13.2g y��18.25% z
$\frac{100}{13.2g}$=$\frac{73}{y��18.25%}$=$\frac{44}{z}$
y=52.8g
z=5.81g
����
�ʴ�Ϊ����1��2.8��
��2��82.5%��
��3��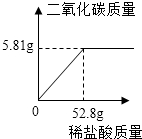 ��
��
��4��18.25%��
���� ������Ҫ����ѧ�����û�ѧ����ʽ�����ʵ�����������ʽ���м����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ�dz��л�ѧ���õ�ʵ����������������и���
��ͼ�dz��л�ѧ���õ�ʵ����������������и����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��˿��������ȼ�գ�2Fe+3O2 $\frac{\underline{\;��ȼ\;}}{\;}$ 2Fe2O3 | |
| B�� | һ����̼��ԭ��������3CO+Fe2O3=2Fe+3CO2 | |
| C�� | ���ܱ�������ȼ�պ�����֤�����غ㶨�ɣ�2P+O2 $\frac{\underline{\;��ȼ\;}}{\;}$ P2O5 | |
| D�� | �ó����ʯ��ˮ���������̼���壺CO2+Ca��OH��2=CaCO3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Щ��Ӧ�����ڻ�����Ӧ | |
| B�� | �����ﶼ�Ǻ��������� | |
| C�� | S�ڷ�Ӧ���б����� | |
| D�� | ������д�Ļ�ѧ��Ӧ�ܹ��������Ǣ٢ڢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �оƾ��Ƽ����Թ���Ĺ���ʱ��Ԥ�� | |
| B�� | ���巢��װ����ʢװҩƷǰ����װ�õ������� | |
| C�� | ���������ʱ������˫����ס������ں��ܲ���ˮ�� | |
| D�� | �����������ƹ���ʹ��С�ձ�ʢװ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ϡ��NaOH��Һ | B�� |  þ�ڿ�����ȼ�� | ||
| C�� |  ������ϡ������ | D�� | 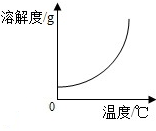 �������ܽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2 | B�� | 3 | C�� | 4 | D�� | 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
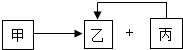 �ס��ҡ����dz��л�ѧ���������ʣ�����һ�������·ֽ���Һͱ���������õ��ܼ�����������ת����ϵ��ͼ��ʾ�����ַ�Ӧ�������ͷ�Ӧ��������ȥ����
�ס��ҡ����dz��л�ѧ���������ʣ�����һ�������·ֽ���Һͱ���������õ��ܼ�����������ת����ϵ��ͼ��ʾ�����ַ�Ӧ�������ͷ�Ӧ��������ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ܢ� | B�� | �٢ۢ� | C�� | �٢ڢۢܢ� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com