
| 34 |
| 98 |
| X |
| 0.4655 |
| 14 |
| 17 |


 »„¶ÆæĪĢĆĻµĮŠ“š°ø
»„¶ÆæĪĢĆĻµĮŠ“š°ø ¼¤»īĖ¼Ī¬ÖĒÄÜѵĮ·æĪŹ±µ¼Ń§Į·ĻµĮŠ“š°ø
¼¤»īĖ¼Ī¬ÖĒÄÜѵĮ·æĪŹ±µ¼Ń§Į·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ĪŖĮĖ²ā¶ØijţÄĢѳʷ֊µ°°×ÖŹµÄŗ¬Į棬ĻÖ²ÉÓĆ”°øĒ¶ū“ļ·Ø”±·Ö½āĘäÖŠµÄµ°°×ÖŹ£®ĘäŌĄķŹĒ°Ńµ°°×ÖŹÖŠµÄµŖŌŖĖŲĶźČ«×Ŗ»Æ³É°±Ęų£Ø»ÆѧŹ½ĪŖ£ŗNH3£©£¬ŌŁÓĆĻ”ĮņĖįĪüŹÕ°±Ęų£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
ĪŖĮĖ²ā¶ØijţÄĢѳʷ֊µ°°×ÖŹµÄŗ¬Į棬ĻÖ²ÉÓĆ”°øĒ¶ū“ļ·Ø”±·Ö½āĘäÖŠµÄµ°°×ÖŹ£®ĘäŌĄķŹĒ°Ńµ°°×ÖŹÖŠµÄµŖŌŖĖŲĶźČ«×Ŗ»Æ³É°±Ęų£Ø»ÆѧŹ½ĪŖ£ŗNH3£©£¬ŌŁÓĆĻ”ĮņĖįĪüŹÕ°±Ęų£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ·Ö½ā |
| ||
| Ė®ÕōĘų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
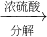 ĮņĖįļ§
ĮņĖįļ§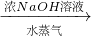 °±£¬ĪŖĮĖ²ā¶ØijţÄĢѳʷ֊µ°°×ÖŹµÄŗ¬Įæ£¬Č”30mLÅ£ÄĢÓĆøĒ¶ū“ļ·Ø·ÖĄėµ°°×ÖŹ£¬°ŃµŖŌŖĖŲĶźČ«×Ŗ»Æ³É°±£ØNH3£©£¬ÓĆ50g4.9%Ļ”H2SO4ČÜŅŗĪüŹÕŗó£¬Ź£ÓąµÄĖįŠčŅŖÓĆ38.0g4.0%µÄNaOHČÜŅŗÖŠŗĶ£¬ĪŹ
°±£¬ĪŖĮĖ²ā¶ØijţÄĢѳʷ֊µ°°×ÖŹµÄŗ¬Įæ£¬Č”30mLÅ£ÄĢÓĆøĒ¶ū“ļ·Ø·ÖĄėµ°°×ÖŹ£¬°ŃµŖŌŖĖŲĶźČ«×Ŗ»Æ³É°±£ØNH3£©£¬ÓĆ50g4.9%Ļ”H2SO4ČÜŅŗĪüŹÕŗó£¬Ź£ÓąµÄĖįŠčŅŖÓĆ38.0g4.0%µÄNaOHČÜŅŗÖŠŗĶ£¬ĪŹ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com