�����ط�ˮ�೧��Ҫ������ʯ��ʯ��ԭ�ϣ�ij��ȤС���ͬѧΪ�˲ⶨij��ʯ��ʯ��̼��Ƶ������������������������ʵ�鷽�����вⶨ��
����I���ٳ�ȡʯ��ʯ10g���ڽ�ʯ��ʯ�����������������ټ���Ϊֹ�����ʲ���Ӧ�����۽����պ�ʣ���������ܱն�F�����������ȴ�����£��ܳƵ�ʣ���������Ϊ6.04g��
��1��������еĻ�ѧ����ʽΪCaCO
3
CaO+CO
2������һ��ѧ��ӦҪ______��������������ա���������
��2��������е����պ�ʣ�����Ϊʲô���ܷ��ڿ�������ȴ��______��
��3������ʱ�ͷų�CO
2������Ϊ______��
�����ٳ�ȡʯ��ʯ��Ʒ10g���ڼ�����������������Ϊ10%��ϡ���ᣬʹ̼�����ȫ��Ӧ�����õ��ܽ����ɵĶ�����̼ͨ�����ʯ��ˮ���������У��ܽ����в����ij������ˡ�ϴ�ӡ�������أ��ó���������Ϊ8.8g���ݼ��㣺
��ʯ��ʯ�к�CaCO
3������Ϊx��
��CaCO
3+2HCl=CaCl
2+CO
2��+H
2O CO
2+Ca��OH��
2=CaCO
3��+H
2O
100 44 44 100
��x=8.8g


8.8g

=88%
��1��������Ľ����88%���뷽��I�Ľ���в�࣬����Ϊ�������ֲ���ԭ�������ʲô����ֻҪ���һ��ԭ��______��
��2������㲽�����������������Ϊ10%���������������Ҫ���٣�

 CaO+CO2������һ��ѧ��ӦҪ______��������������ա���������
CaO+CO2������һ��ѧ��ӦҪ______��������������ա���������
 8.8g
8.8g =88%
=88%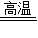 CaO+CO2������һ��ѧ��ӦҪ�����������ʴ�Ϊ�����գ�
CaO+CO2������һ��ѧ��ӦҪ�����������ʴ�Ϊ�����գ�


 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�
 CaO+CO2������һ��ѧ��ӦҪ________��������������ա���������
CaO+CO2������һ��ѧ��ӦҪ________��������������ա���������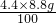 ������
������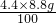 ����8.8g
����8.8g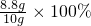 =88%
=88%