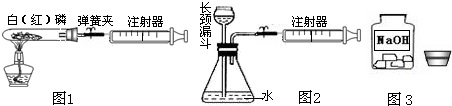
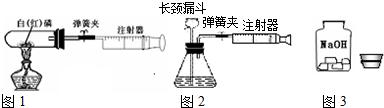
�⣺��1������ȼ�ջ�ɰ�ɫ�������������ף��ʿɹ۲쵽�����������̣����ڰ���ȼ�����ľ���������

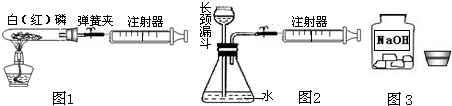
�����������װ���������������50mL��

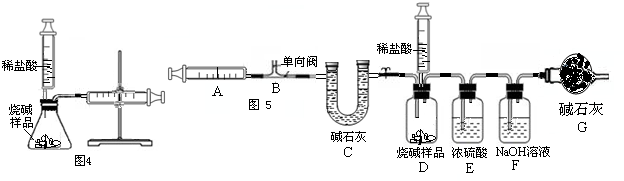
=10mL������ע������10mL������벹�䣬ע������ʣ���������20mL-10mL=10mL����ˣ����Կ��������������Ƶ�Լ10mL������ʵ���ٴ���֤�˿�����Լ��

��21%��������
��2����������������ע�����Ļ���ʱ����ƿ�����屻����ע���������װ�����������ã����Ŀ����ͻ�ӳ���©�����벹�䣬��ˣ��ɹ۲쵽����©���¶˹ܿڲ������ݣ���ѡD��
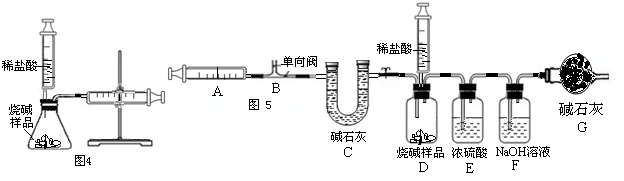
��3����I��ȡ��ҩƷʱ���Լ�ƿ��ƿ��Ӧ�����������ϣ��ڱ�����������ʱ�������������Ƽ������տ����е�ˮ�����⣬�������տ����еĶ�����̼�����ʣ���˱����ܷⱣ�棻
��II���������������ᷢ���кͷ�Ӧ�����Ȼ��ƺ�ˮ����ѧ����ʽΪNaOH+HCl=NaCl+H
2O��̼���������ᷢ�����ֽⷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼���壬��ѧ����ʽΪNa
2CO
3+2HCl=2NaCl+H
2O+CO
2������Ϊ��ѡ�õ�������лӷ��ԣ����ò����Ķ�����̼���������ⶨ��ȷ�������ɶ�̼�������������ⶨ��ȷ����ѡ��������ݻ����ޣ��������϶�����ʱ���������������ѡע��������ʱ���������������ʵ��ʧ�ܣ����ԣ�����ҩƷʱҪ�ʵ����п��ƣ�
��III����i������װ��E��Ũ���������ˮ�ԣ���װ����ʵ������ҪΪ��ȥ������̼�е�ˮ������װ��G�еļ�ʯ�Ҽ������տ����е�ˮ���������տ����еĶ�����̼��������װ��F�����֮�䣬�������ڷ�ֹ�����еĶ�����̼��ˮ��������F�У�
��ii������ע������ʹװ���ڲ����Ķ�����̼��ȫ��װ��F������������Һ���գ��������ٶȹ��죬����ɶ�����̼����δ�����ն��ų���ʹ�òⶨ�Ķ�����̼����ƫС�����������ⶨ���ƫС��
��iii������װ��F�������仯����֪��Ӧ���ɶ�����̼������=321.6g-320.5g=1.1g������Ʒ��̼���Ƶ�����Ϊx
Na
2CO
3+2HCl=2NaCl+H
2O+CO
2��
106 44
x 1.1g
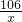
=
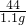
x=2.65g
���ռ���Ʒ��Na
2CO
3����������=
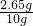
��100%=26.5%
�ʴ�Ϊ����1�������������̣�10��

��21%��
��2��D��
��3����I�������������ƹ�����ˮ���⣻���������������̼��Ӧ���ʣ�
��II��NaOH+HCl=NaCl+H
2O��Na
2CO
3+2HCl=2NaCl+H
2O+CO
2���������Ķ�����̼�л���ˮ�������Ȼ������壻����Ʒȡ���ˣ�����������ܳ���ע�������̣�����Ʒȡ���ˣ���������̫�٣���������
��III����i����ȥ������̼�е�ˮ��������ֹ�����еĶ�����̼��ˮ��������F�У���ii��ƫС����iii��26.5%��
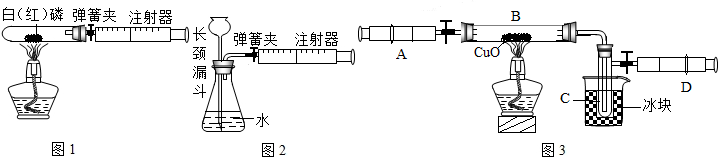
��������1���������ڿ�����ȼ�����ľ������ɲⶨ����ռ�������

��ʵ������������⣻
��2������ʵ���Ҽ��װ�������Ե�ԭ����������װ���ڽ��������Լ���ʱ���ܳ��ֵ�����
��3����I������ʵ���Ҷ�ҩƷȡ��ʱ��Ҫ���������Ƶ����ʣ�����ͼʾ�����д��ڵIJ����ϲ���Ҫ������⣻
��II����������������̼�����������ᷴӦ�����ʣ�д��������Ӧ�Ļ�ѧ����ʽ�������ѡ��ҩƷ��װ�ã��Բ����п��ܳ��ֵĽ�����з����������жϣ�
��III����i�����ݶ�����װ�ü�ʵ��Ŀ�����⣬���װ��������ҩƷ�����ԣ��ж�ʢ��Ũ�����װ��E��ʢ�ż�ʯ�ҵ�װ��G�����ã�
��ii����������ע����������ʵ������ã��Բ�ǡ�����������з������жϸò��������Բⶨ�����Ӱ�죻
��iii��ʵ�����ݵĴ���������װ��F�������仯�ó���Ӧ����������̼��������������һ���������ݷ�Ӧ�Ļ�ѧ����ʽ������Ʒ��̼���Ƶ��������Ӷ���������ռ���Ʒ��Na
2CO
3������������
�����������������ɶ�����̼��������ʱ������ʹ����ȫ��Ӧ�������321.6g�뿪ʼ����ҩƷ������320g����������ã���Ϊ����ʵ������и�װ�û�������װ�ÿ����еĶ�����̼�����պ�������Ϊ320.5g��

 �����������װ���������������50mL��
�����������װ���������������50mL�� =10mL������ע������10mL������벹�䣬ע������ʣ���������20mL-10mL=10mL����ˣ����Կ��������������Ƶ�Լ10mL������ʵ���ٴ���֤�˿�����Լ��
=10mL������ע������10mL������벹�䣬ע������ʣ���������20mL-10mL=10mL����ˣ����Կ��������������Ƶ�Լ10mL������ʵ���ٴ���֤�˿�����Լ�� ��21%��������
��21%��������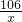 =
=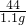 x=2.65g
x=2.65g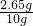 ��100%=26.5%
��100%=26.5% ��21%��
��21%�� ��ʵ������������⣻
��ʵ������������⣻





 ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����
ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����