£Ø2006?ŠžĪäĒųŅ»Ä££©ĖįÓźŹĒpHŠ”ÓŚ5.6µÄ½µĖ®£®ĪŅ¹ś“ó²æ·ÖµŲĒųĖł½µĖįÓźÖ÷ŅŖŹĒÓɾÓĆńŗĶ¹¤³§Č¼ÉÕŗ¬ĮņµÄĆŗŅŌ¼°Ä³Š©»Æ¹¤³§Éś²ś¹ż³ĢÖŠÅŷŵĶžŃõ»ÆĮņĘųĢ壬¾¹żŅ»ĻµĮŠ»Æѧ·“Ó¦¶ųŠĪ³ÉµÄ£®
£Ø1£©Čē¹ūĮņ·ŪŌŚŃõĘųÖŠČ¼ÉÕ£¬¹Ū²ģµ½µÄĻÖĻóŹĒ
²śÉśĆ÷ĮĮµÄĄ¶×ĻÉ«»šŃę£¬·Å³öČČĮ棬²śÉśŅ»ÖÖĪŽÉ«ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢå
²śÉśĆ÷ĮĮµÄĄ¶×ĻÉ«»šŃę£¬·Å³öČČĮ棬²śÉśŅ»ÖÖĪŽÉ«ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢå
£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£®
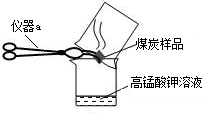
£Ø2£©¼øĪ»Ķ¬Ń§ĪŖĮĖĢ½¾æľĢæÖŠŹĒ·ńŗ¬ÓŠÉŁĮæĮņŌŖĖŲ£¬ĖūĆĒÉč¼ĘĮĖČēĶ¼ĖłŹ¾ŹµŃé½ųŠŠ²ā¶Ø£®ĒėŠ“³öĶ¼ÖŠŅĒĘ÷aµÄĆū³Ę£ŗ
ŪįŪöĒÆ
ŪįŪöĒÆ
£®ĖūĆĒ²éŌÄ׏ĮĻŗóµĆÖŖ£ŗ”°¶žŃõ»ÆĮņÄÜŹ¹KMnO
4ČÜŅŗĶŹÉ«£ØÓÉ×ĻŗģÉ«±ä³ÉĪŽÉ«£©£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗ
”°5SO
2+2KMnO
4+2H
2OØTK
2SO
4+2MnSO
4+2

”±£®Č»¶ų»Æѧ·½³ĢŹ½ÖŠ×īŗóŅ»ÖÖĪļÖŹµÄ»ÆѧŹ½Ó”Ė¢²»Ē峞£¬øł¾ŻÉĻĻĀĪÄĮĖ½āµ½øĆĪļÖŹŹĒŅ»ÖÖĖį£¬Ēėøł¾ŻŅŃѧÖŖŹ¶ĶĘ²āĘä»ÆѧŹ½£ŗ
H2SO4
H2SO4
£®·“Ó¦ŗóÉś³ÉµÄMnSO
4ÖŠMnŌŖĖŲ»ÆŗĻ¼ŪĪŖ
+2
+2
£®
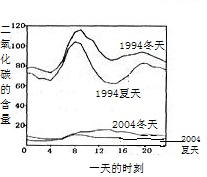
£Ø3£©ČēĶ¼ŹĒ1994ÄźŗĶ2004ÄźÄ³³ĒŹŠµÄŅ»Ģģø÷øöŹ±æĢ²āµ½µÄæÕĘųÖŠ¶žŃõ»ÆĮņµÄŗ¬Į森ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ
D
D
A£®ĒśĻßĻŌŹ¾¶¬Ģģ“óĘųÖŠµÄ¶žŃõ»ÆĮņŗ¬Įæ±ČĻÄĢģøß
B£®ĒśĻßĻŌŹ¾ĮĖ1994ÄźŅ»ĢģÖŠ“óŌ¼8µć×óÓŅ¶žŃõ»ÆĮņµÄŗ¬Įæ½Ļøß
C£®¶žŃõ»ÆĮņµÄŗ¬ĮæŌŚ10Äź¼ä½µµĶµÄŌŅņæÉÄÜŹĒ¼ÓĒæĮĖČ¼ĮĻµÄĶŃĮņŗĶæŲÖĘĮĖ¶žŃõ»ÆĮņµÄÅÅ·Å
D£®¶žŃõ»ÆĮņĪŪČ¾µÄÖ÷ŅŖĄ“Ō“ŹĒĘū³µÅŷŵÄĪ²Ęų£¬æŲÖĘĪŪČ¾µÄ·½·ØŹĒ½ūÖ¹Ź¹ÓĆĘū³µ
£Ø4£©ŌŚŃŠ¾æĖįÓźĪ£ŗ¦µÄ¹ż³ĢÖŠ£¬²ā¶ØøƵŲĒųÓźĖ®µÄpHµÄŹµŃé²Ł×÷
ÓĆ²£Į§°ōÕŗȔӟĖ®£¬µćŌŚŹŌÖ½ÉĻ£¬Óė±ź×¼±ČÉ«æضŌÕÕ£¬¶ĮČ”ŹżÖµ
ÓĆ²£Į§°ōÕŗȔӟĖ®£¬µćŌŚŹŌÖ½ÉĻ£¬Óė±ź×¼±ČÉ«æضŌÕÕ£¬¶ĮČ”ŹżÖµ
£®
ij»ÆѧŠĖȤŠ”×éČ”øÕ½µµ½µŲĆęµÄÓźĖ®£¬ĆæøōŅ»¶ØŹ±¼äÓĆ½Ļ¾«ĆܵÄpH¼Ę²ā¶ØĘäpH£¬Źż¾ŻČēĻĀ£ŗ
| ²ā¶ØŹ±¼ä/·ÖÖÓ |
0 |
1 |
2 |
3 |
| pH |
4.73 |
4.62 |
4.56 |
4.55 |
ÓÉ“ĖæÉÖŖøĆÓźĖ®µÄĖįŠŌŌ½Ą“Ō½
Ēæ
Ēæ
£ØĢī”°Ēæ”±»ņ”°Čõ”±£©£¬×īÖÕĒ÷ÓŚĪČ¶Ø£®
£Ø5£©ĖįÓźŌģ³ÉµÄĪ£ŗ¦ŗܶą£®Ä³ŠĖȤŠ”×éµ÷²é·¢ĻÖÄĻ¾©µÄĮł³ÆŹÆæĢµÄ±ķĆęÓŠ²»Ķ¬³Ģ¶ČµÄøÆŹ“£®ĒŅ½ü20ÄźµÄøÆŹ“ĖŁ¶Č“ó“󳬹żŅŌĶł£¬ĘäÖŠ×īÖ÷ŅŖŌŅņÖ®Ņ»¾ĶŹĒĖįÓź£®ĪŖĮĖ¼õ»ŗŹÆæĢøÆŹ“£¬ĒėÄćĢįŅ»Ģõ½ØŅé£ŗ
Ź¹ÓĆĒå½ąÄÜŌ“
Ź¹ÓĆĒå½ąÄÜŌ“
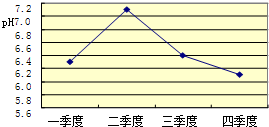
£® ĖįÓź»įŹ¹ŗÓĮ÷”¢ŗž²“Ėį»Æ£®ĻĀĶ¼ŹĒijŹŠČ„ÄźÄ³ŗÓĮ÷Ņ»ÄźÖŠ²»Ķ¬Ź±ĘŚŗÓĖ®µÄĘ½¾łpH±ä»ÆµÄÕŪĻßĶ¼£®ŌņČ„ÄźŗÓĖ®ĖįŠŌ×īĒæµÄ¼¾¶ČŹĒµŚ
ĖÄ
ĖÄ
¼¾¶Č£®øĆ¼¾¶ČÓźĖ®ĖįŠŌ×īĒæµÄŌŅņæÉÄÜŹĒ£ŗ£Ø“šĮ½Ģõ£©
¶¬¼¾Č”ÅÆ£¬ŠčŅŖČ¼ÉÕ“óĮæ»ÆŹÆČ¼ĮĻ£¬¼Ó“óĮĖ¶ŌŗÓĖ®µÄĪŪČ¾£»¶¬¼¾½µĖ®Įæ¼õÉŁ£¬ŗÓĖ®ÖŠĖįµÄÅضČŌö“óµČ
¶¬¼¾Č”ÅÆ£¬ŠčŅŖČ¼ÉÕ“óĮæ»ÆŹÆČ¼ĮĻ£¬¼Ó“óĮĖ¶ŌŗÓĖ®µÄĪŪČ¾£»¶¬¼¾½µĖ®Įæ¼õÉŁ£¬ŗÓĖ®ÖŠĖįµÄÅضČŌö“óµČ
£®
ĖįÓź»¹»įŹ¹ĶĮČĄĖį»Æ£®ĪŖĮĖÖŠŗĶĖįŠŌĶĮČĄ£¬æÉŅŌŹ¹ÓĆŹģŹÆ»Ņ·ŪÄ©£¬ČōøĆĖįÓźµÄ³É·ÖŹĒĮņĖį£¬ĒėŠ“³öøĆÖŠŗĶ·“Ó¦µÄ»Æѧ·½³ĢŹ½
H2SO4+Ca£ØOH£©2=CaSO4+2H2O
H2SO4+Ca£ØOH£©2=CaSO4+2H2O
£®
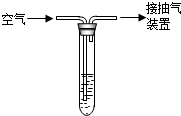
£Ø6£©Ä³Š£»ÆѧŠĖȤŠ”×éŌŚŃ§Ķź¼īµÄ»ÆѧŠŌÖŹŗó£¬Ļėµ½ÓĆNaOHČÜŅŗĪüŹÕSO
2£¬·“Ó¦»Æѧ·½³ĢŹ½ČēĻĀ£ŗ2NaOH+SO
2ØTNa
2SO
3+H
2O ÓĆNaOHČÜŅŗĪüŹÕ1000LŅŃ³żČ„CO
2µÄæÕĘųѳʷ£¬ČÜŅŗÖŹĮæŌöÖŲĮĖ0.64g£®ŅŃÖŖ“ĖŹ±æÕĘųµÄĆܶČŌ¼ĪŖ1.3g/L£¬Ēó£ŗ
¢Ł±»ĪüŹÕµÄSO
2µÄÖŹĮæ
0.64g
0.64g
g£®
¢Ś·¢Éś·“Ó¦µÄNaOHµÄÖŹĮ森£ØĻą¶ŌŌ×ÓÖŹĮæ£ŗNa-23 S-32 O-16£©
¢ŪæÕĘųÖŠSO
2µÄÖŹĮæ·ÖŹż£Ø¼ĘĖć½į¹ū¾«Č·µ½0.01%£©£®

 ŗ¬ĮņµÄĪļÖŹÓŠŗܶą£¬ĘäÖŠÓŠ³õÖŠ»Æѧ֊³£¼ūµÄĪļÖŹ£®
ŗ¬ĮņµÄĪļÖŹÓŠŗܶą£¬ĘäÖŠÓŠ³õÖŠ»Æѧ֊³£¼ūµÄĪļÖŹ£® 2Fe2O3+8SO2£¬µŚ¶ž²½£ŗ2SO2+O2
2Fe2O3+8SO2£¬µŚ¶ž²½£ŗ2SO2+O2 2SO3£¬µŚČż²½£ŗSO3+H2O=H2SO4£®µŚŅ»²½·“Ó¦ĖłŹōµÄ·“Ó¦ĄąŠĶĪŖ________£ØĢīŠņŗÅ£©£®
2SO3£¬µŚČż²½£ŗSO3+H2O=H2SO4£®µŚŅ»²½·“Ó¦ĖłŹōµÄ·“Ó¦ĄąŠĶĪŖ________£ØĢīŠņŗÅ£©£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø


 ”±£®Č»¶ų»Æѧ·½³ĢŹ½ÖŠ×īŗóŅ»ÖÖĪļÖŹµÄ»ÆѧŹ½Ó”Ė¢²»Ē峞£¬øł¾ŻÉĻĻĀĪÄĮĖ½āµ½øĆĪļÖŹŹĒŅ»ÖÖĖį£¬Ēėøł¾ŻŅŃѧÖŖŹ¶ĶĘ²āĘä»ÆѧŹ½£ŗ
”±£®Č»¶ų»Æѧ·½³ĢŹ½ÖŠ×īŗóŅ»ÖÖĪļÖŹµÄ»ÆѧŹ½Ó”Ė¢²»Ē峞£¬øł¾ŻÉĻĻĀĪÄĮĖ½āµ½øĆĪļÖŹŹĒŅ»ÖÖĖį£¬Ēėøł¾ŻŅŃѧÖŖŹ¶ĶĘ²āĘä»ÆѧŹ½£ŗ
 Įņ»ņŗ¬ĮņĪļÖŹŌŚæÕĘųÖŠČ¼ÉÕÉś³ÉSO2£¬SO2ŹĒŅ»ÖÖĪŽÉ«”¢ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄÓŠ¶¾ĘųĢ壮
Įņ»ņŗ¬ĮņĪļÖŹŌŚæÕĘųÖŠČ¼ÉÕÉś³ÉSO2£¬SO2ŹĒŅ»ÖÖĪŽÉ«”¢ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄÓŠ¶¾ĘųĢ壮