
 Ä³ŃŠ¾æŠŌѧĻ°Š”×éŌŚŠÖśĄĻŹ¦ĒåĄķŹµŃéŹŅŹ±£¬·¢ĻÖŅ»Åś“ę·Å¶ąÄźµÄĒāŃõ»ÆøĘ£®ĪŖ¼ģŃéĘä±äÖŹĒéæö£¬½ųŠŠĮĖČēĻĀĢ½¾æ£ŗČ”ĒāŃõ»ÆøĘѳʷ11.4gӌ׶ŠĪĘæÖŠ£¬¼ÓČė38.6gĖ®£¬Õńµ“ŠĪ³ÉŠü×ĒŅŗ£¬·ÅŌŚµē×ÓĢģĘ½ÉĻ£¬Ļņ׶ŠĪĘæÖŠÖšµĪµĪ¼Ó14.6%µÄĻ”ŃĪĖį£¬ČēĶ¼¼×ĖłŹ¾£¬Õńµ“ŗó¶ĮȔ֏Į森µĆµ½×¶ŠĪĘæÖŠĪļÖŹµÄÖŹĮæÓėĻ”ŃĪĖįÖŹĮæ¹ŲĻµ£¬ČēĶ¼ŅŅĖłŹ¾£¬ŹŌĒó£ŗ
Ä³ŃŠ¾æŠŌѧĻ°Š”×éŌŚŠÖśĄĻŹ¦ĒåĄķŹµŃéŹŅŹ±£¬·¢ĻÖŅ»Åś“ę·Å¶ąÄźµÄĒāŃõ»ÆøĘ£®ĪŖ¼ģŃéĘä±äÖŹĒéæö£¬½ųŠŠĮĖČēĻĀĢ½¾æ£ŗČ”ĒāŃõ»ÆøĘѳʷ11.4gӌ׶ŠĪĘæÖŠ£¬¼ÓČė38.6gĖ®£¬Õńµ“ŠĪ³ÉŠü×ĒŅŗ£¬·ÅŌŚµē×ÓĢģĘ½ÉĻ£¬Ļņ׶ŠĪĘæÖŠÖšµĪµĪ¼Ó14.6%µÄĻ”ŃĪĖį£¬ČēĶ¼¼×ĖłŹ¾£¬Õńµ“ŗó¶ĮȔ֏Į森µĆµ½×¶ŠĪĘæÖŠĪļÖŹµÄÖŹĮæÓėĻ”ŃĪĖįÖŹĮæ¹ŲĻµ£¬ČēĶ¼ŅŅĖłŹ¾£¬ŹŌĒó£ŗ·ÖĪö øł¾ŻĒāŃõ»ÆøĘĪüŹÕæÕĘųÖŠµÄ¶žŃõ»ÆĢ¼Éś³ÉĢ¼Ėįøʶų±äÖŹ£¬Ļņ±äÖŹµÄĒāŃõ»ÆøʵÄ×ĒŅŗÖŠµĪ¼ÓŃĪĖį£¬ŃĪĖįĻČÓėĒāŃõ»ÆøĘ·“Ó¦£¬Éś³ÉĀČ»ÆøĘŗĶĖ®£¬¶ų²»ÄܲśÉśĘųĢ壬Ņņ“ĖĖęŃĪĖįµÄµĪ¼ÓĘæÄŚÖŹĮæ²»¶ĻŌö¼Ó£¬ČēĶ¼ŅŅÖŠµÄµŚŅ»¶ĪĒśĻßĖłŹ¾£»µ±ĒāŃõ»ÆøĘĶźČ«·“Ó¦ŗ󣬼ĢŠųµĪ¼ÓµÄŃĪĖįæŖŹ¼ÓėĢ¼ĖįøĘ·“Ó¦£¬¼““ÓĶ¼ŅŅĘæÄŚÖŹĮæĪŖ100gŹ±Ź¼£¬Ģ¼ĖįøĘÓėŃĪĖį·“Ӧɜ³ÉĀČ»ÆøĘŗĶĖ®£¬Ķ¬Ź±·Å³öĘųĢ嶞Ńõ»ÆĢ¼£¬Ņņ“ĖĖęŃĪĖįµÄµĪ¼ÓĘæÄŚĪļÖŹÖŹĮæŌö¼ÓĒéæöČēĶ¼ŅŅÖŠµÄµŚ¶ž¶ĪĒśĻߣ»ÖĮĢ¼ĖįøĘĶźČ«·“Ó¦ŗ󣬵Ī¼ÓµÄŃĪĖį²»·¢Éś·“Ó¦£¬ĘæÄŚÖŹĮæŌö¼ÓĒéæöČēĶ¼ŅŅÖŠµŚČż¶ĪĒśĻߣ»øł¾ŻÓėĒāŃõ»ÆøĘ½×¶Ī·“Ó¦µÄ»Æѧ·½³ĢŹ½¼°ĻūŗÄŃĪĖįµÄÖŹĮ棬æɼĘĖć³ö²Ī¼Ó·“Ó¦µÄĒāŃõ»ÆøʵÄÖŹĮ棬Ģ¼ĖįøʵÄÖŹĮæĪŖѳʷÓėĒāŃõ»ÆøʵÄÖŹĮæ²īŅŌ¼°Ģ¼ĖįøĘĻūŗÄŃĪĖįµÄÖŹĮæ½ųŠŠ·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©ÓÉĢāŅāŗĶĶ¼Ļó·ÖĪöÖŖ£¬µŚŅ»½×¶Ī׶ŠĪĘæÖŠĪļÖŹŌö¼ÓµÄÖŹĮæ¾ĶŹĒÓėѳʷ֊ĒāŃõ»ÆøĘ·“Ó¦µÄŃĪĖįµÄÖŹĮ棬µŚŅ»½×¶ĪµĪČėŃĪĖįµÄÖŹĮæĪŖ100g-50g=50g
ÉčÓėŃĪĖį·“Ó¦µÄCa£ØOH£©2µÄÖŹĮæĪŖx£¬Éś³ÉĀČ»ÆøĘĪŖa
Ca£ØOH£©2+2HCl=CaCl2+2H2O
74 73 111
x 50g”Į14.6% a
$\frac{74}{x}$=$\frac{73}{50g”Į14.6%}$=$\frac{111}{a}$
½āµĆx=7.4g£¬a=11.1g
ŹōÓŚ»ģŗĻĪļÖŠĢ¼ĖįøʵÄÖŹĮæĪŖ£ŗ11.4g-7.4g=4g£»
£Ø2£©4gĢ¼ĖįøĘĻūŗÄŃĪĖįµÄÖŹĮæĪŖy£¬Éś³É¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖz£¬Éś³ÉĀČ»ÆøĘĪŖb£¬
CaCO3+2HClØTCaCl2+H2O+CO2”ü
100 73 111 44
4g y”Į14.6% a z
$\frac{100}{4g}$=$\frac{73}{y”Į14.6%}$=$\frac{44}{z}$=$\frac{111}{a}$
y=20g
z=1.76g
a=4.44g
yÖįÉĻµÄBµćĖł±ķŹ¾×¶ŠĪĘæÖŠµÄĪļÖŹÖŹĮæ=100g+20g-1.76g=118.24g£¬
BµćŹ±ĖłµĆČÜŅŗµÄČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ£ŗ$\frac{11.1g+4.44g}{118.24g}$”Į100%=13.1%£®
“š£ŗ£Ø1£©“Ėѳʷ֊ĒāŃõ»ÆøʵÄÖŹĮæŹĒ7.4g£¬Ģ¼ĖįøʵÄÖŹĮæŹĒ4g£»
£Ø2£©ŌŚBµćŹ±ĖłµĆČÜŅŗµÄČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ13.1%£®
µćĘĄ øł¾ŻĪļÖŹ¼ä·“Ó¦µÄĻČŗó¹ŲĻµ£¬·ÖĪö·“Ó¦¶ŌČŻĘ÷ÄŚĪļÖŹÖŹĮæµÄÓ°Ļģ£¬µĆ³öĖł¼ÓČėČÜŅŗµÄÖŹĮæ£»Č»ŗóøł¾Ż·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬Ķź³É·ÖĪöÓė¼ĘĖć£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ĪļÖŹ | ±½ | ŃõĘų | ¶žŃõ»ÆĢ¼ | Ė® | X |
| ·“Ó¦Ē°ÖŹĮæ/g | 3.9 | 9.6 | 0 | 0 | 0 |
| ·“Ó¦ŗóÖŹĮæ/g | 0 | 0 | 6.6 | 2.7 | m |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŹµŃé“ĪŹż | ¼ÓČėĻ”ŃĪĖįµÄÖŹĮæ/g | Ź£Óą¹ĢĢåµÄÖŹĮæ/g |
| 1 | 20 | 11 |
| 2 | 20 | 6 |
| 3 | 20 | 2.8 |
| 4 | 20 | n |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | K2MnO4”¢MnO2 | B£® | KMnO4”¢MnO2 | ||
| C£® | KMnO4”¢K2MnO4”¢MnO2 | D£® | KMnO4”¢K2MnO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
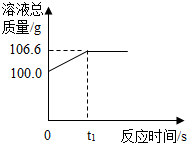 ĪŖ²ā¶Øij³ąĢśæóŹÆÖŠŃõ»ÆĢśµÄÖŹĮæ·ÖŹż£¬Š”ĮśŗĶĖūµÄĶ¬Ń§ÓĆ×ćĮæµÄŅ»Ńõ»ÆĢ¼Óė10g³ąĢśæóŹÆѳʷ³ä·Ö·“Ó¦£ØŌÓÖŹ²»²ĪÓė·“Ó¦£©£¬²¢½«Éś³ÉµÄĘųĢåÓĆŅ»¶ØĮæµÄĒāŃõ»ÆÄĘČÜŅŗĶźČ«ĪüŹÕ£¬øĆČÜŅŗ×ÜÖŹĮæÓė·“Ó¦Ź±¼äµÄ±ä»Æ¹ŲĻµČēĶ¼£®
ĪŖ²ā¶Øij³ąĢśæóŹÆÖŠŃõ»ÆĢśµÄÖŹĮæ·ÖŹż£¬Š”ĮśŗĶĖūµÄĶ¬Ń§ÓĆ×ćĮæµÄŅ»Ńõ»ÆĢ¼Óė10g³ąĢśæóŹÆѳʷ³ä·Ö·“Ó¦£ØŌÓÖŹ²»²ĪÓė·“Ó¦£©£¬²¢½«Éś³ÉµÄĘųĢåÓĆŅ»¶ØĮæµÄĒāŃõ»ÆÄĘČÜŅŗĶźČ«ĪüŹÕ£¬øĆČÜŅŗ×ÜÖŹĮæÓė·“Ó¦Ź±¼äµÄ±ä»Æ¹ŲĻµČēĶ¼£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ŹŌ¹ÜĘĘĮŃ | B£® |  ĮæČ”µÄŅŗĢåĘ«ÉŁ | C£® |  ĻšĘ¤ČūµÆ³ö | D£® |  µĘÄŚ¾Ę¾«Č¼ÉÕ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com