
| ||
| ||
| ||
| ||



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
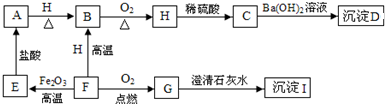
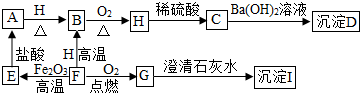
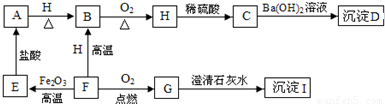
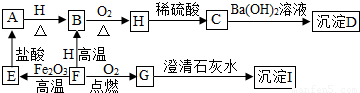
A”¢B”¢C”¢D”¢E”¢F”¢G”¢H”¢I¶¼ŹĒ³õÖŠ»Æѧ֊³£¼ūµÄĪļÖŹ”£ĘäÖŠE”¢F”¢H¾łĪŖŗŚÉ«¹ĢĢ壬BŹĒ×ĻŗģÉ«¹ĢĢ壬DŹĒ»ģŗĻĪļ”£ĖüĆĒÖ®¼äÓŠČēĻĀµÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·ÖÉś³ÉĪļŅŃĀŌČ„£¬ŅŃÖŖĒāĘųŌŚŅ»¶ØĢõ¼žĻĀÄÜ»¹ŌŃõ»ÆĶ£©£ŗ
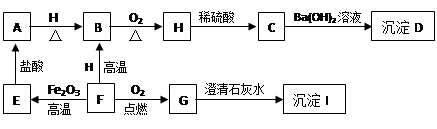
£Ø1£© Š“³öDĪļÖŹµÄ×é³É£ŗ___________”¢________________”£
£Ø2£© Š“³öA”¢HĪļÖŹµÄ»ÆѧŹ½£ŗA________________£¬H ________________”£
£Ø3£©G”Ŗ”śI µÄ»Æѧ·½³ĢŹ½ŹĒ________________________________________”£
£Ø4£©F”Ŗ”śE µÄ»Æѧ·½³ĢŹ½ŹĒ________________________________________”£
£Ø5£©Ėµ³öBĪļÖŹµÄŅ»ÖÖÓĆĶ¾£ŗ________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2013Äź¹ć¶«Ź”ÖŠæ¼»ÆŃ§Ä£ÄāŹŌ¾ķ£Ø¶ž£©£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźøŹĖąŹ”Ę½Į¹ŹŠ”¢ĒģŃōŹŠÖŠæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com