
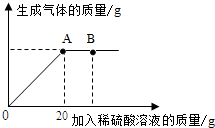
 ��һ�ձ���ʢ��25g��Na2CO3��Na2SO4���壬����������ˮ�Ƴɲ�������Һ�������еμ����ʵ��ʾ�����Ϊ9.8%��H2SO4��Һ�����������������������H2SO4��Һ��������ϵ������ͼ��ʾ�����������ش��������⣺
��һ�ձ���ʢ��25g��Na2CO3��Na2SO4���壬����������ˮ�Ƴɲ�������Һ�������еμ����ʵ��ʾ�����Ϊ9.8%��H2SO4��Һ�����������������������H2SO4��Һ��������ϵ������ͼ��ʾ�����������ش��������⣺���� ��1������̼������ϡ���ᷴӦ��������з�����
��2����ͼʾ���ݿ�֪��������ϡ������ͼ��B��ʱ����������ȫ��Ӧ������������ݴ˷�����
��3����ͼʾ���ݿ�֪��������ϡ������A��ʱ�����岻�����ɣ�˵��̼������ȫ��Ӧ������̼���ƺ�ϡ���ᷴӦ�Ļ�ѧ����ʽ��������������г�����ʽ���Ϳɼ������������������Ȼ����ݡ����ò�������Һ������=���뷴Ӧ����Һ����-�����������������㣮
��� �⣺��1��̼������ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��Na2CO3+H2SO4�TNa2SO4+H2O+CO2�����ɴ˿��Եó����ڵ���ϡ����ʱ���۲쵽������ʵ�������ǣ��������ʲ����ܽ⣻�������������������ð������
��2����ͼʾ���ݿ�֪��������ϡ������ͼ��B��ʱ����������ȫ��Ӧ�������������ʱ�ձ�����Һ�ﺬ�е�������Na2SO4��H2SO4��
��3��20g9.8%��ϡ�����к�����������ǣ�20g��9.8%=1.96g��
�跴Ӧ�����ɶ�����̼������Ϊx��
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
98 44
1.96g x
$\frac{98}{1.96g}$=$\frac{44}{x}$
��ã�x=0.88g
��ʱ���ò�������Һ�����ʵ�����Ϊ��25g+20g-0.88g=44.12g
�𣺴�ʱ���ò�������Һ�����ʵ�����Ϊ44.12g��
�ʴ�Ϊ����1���������ʲ����ܽ⣬������ð����
��2��Na2SO4��H2SO4��
��3��44.12g��
���� ������Ҫ����ѧ�����û�ѧ����ʽ���м��������������Ĺؼ��ǽ�����ʼ䷴Ӧ���������ȷ����ͼʾ���ݣ�ϸ�Ľ��


 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������£�̼�������̼������Ӧ����һ�����ȷ�Ӧ | |
| B�� | ������ʯ��ˮ�в���ͨ��һ����̼��ʯ��ˮ������ | |
| C�� | ��ƽ��ѧ����ʽ�������Ƿ�Ӧǰ��ԭ���������Ŀ���� | |
| D�� | ���ʯ��ʯī������̼ԭ��ֱ�ӹ��ɵĵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���� | ʾ�� |
| A | ʵ�鷨 | �ú��������ⶨ������������������ʵ�� |
| B | ���෨ | ����������ʵ�Ԫ�����࣬���������Ϊ���ʺͻ����� |
| C | ���ɷ� | ˮ��������̼�Ƿ��ӹ��ɵģ����ɳ��������ʶ����ɷ��ӹ��ɵ� |
| D | �Աȷ� | �õ������ˮ�Ϳ�������ѹ�����ó�������Һ������ļ�϶��С��ϵ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
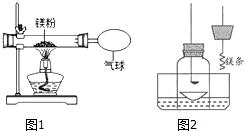 ij��ȤС��Ϊ��֤�����غ㶨�ɣ�����þ���ڿ�����ȼ�յ�ʵ�飮
ij��ȤС��Ϊ��֤�����غ㶨�ɣ�����þ���ڿ�����ȼ�յ�ʵ�飮| ʵ����� | ʵ�������� |
| ��һ�����ĵ���þ�����Թ��У�Ȼ�����������ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� | �д�������ð����ʪ��ĺ�ɫʯ����ֽ��������˻�ɫ������Mg3N2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com