
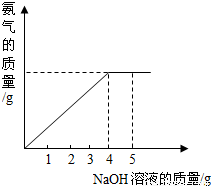
 ×100%��
×100%��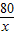 =
=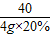 ��=1.6g
��=1.6g  =
=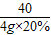 y=1.7g
y=1.7g 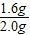 ×100%=80%
×100%=80%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2010?�عأ�С���ͼ�λͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ�����ձ��е�����������Һ�μ�ϡ����һ������������˵μ�ָʾ������������ֹͣʵ�飬����ʱ�ձ�����Һ�е����ʵijɷ�չ���˱��ۣ�
��2010?�عأ�С���ͼ�λͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ�����ձ��е�����������Һ�μ�ϡ����һ������������˵μ�ָʾ������������ֹͣʵ�飬����ʱ�ձ�����Һ�е����ʵijɷ�չ���˱��ۣ�| ʵ�鲽�� | Ԥ������ | Ԥ�ƽ��� |
| ȡ������Ӧ�����Һ���Թ��У���μ���̼������Һ�� | �������ȷ | |
| �������ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2010?�عأ����ִ������У�����Խ��Խע����Ԫ����ȡ����Ԫ�ض���������������Ҫ�����ã���ͼ��ij���г����۵�һ�֡��ӵ�ʳ�Ρ���װ���ϵIJ���˵����
��2010?�عأ����ִ������У�����Խ��Խע����Ԫ����ȡ����Ԫ�ض���������������Ҫ�����ã���ͼ��ij���г����۵�һ�֡��ӵ�ʳ�Ρ���װ���ϵIJ���˵�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ�����н���������һ���п���ѧģ���Ծ��������������棩 ���ͣ������
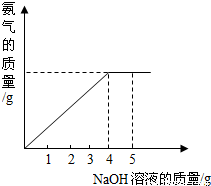
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com