

| װ�ñ� | װ�ö� | |
| ��Ӧǰ | 48.8�� | 161.2�� |
| ��Ӧ�� | 46.5�� | 163.9�� |
 Cu+H2O
Cu+H2O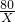 =
=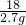
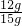 ��100%=80%
��100%=80%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鲽�� | ʵ������ | ���ۺͻ�ѧ����ʽ |
| ����ͬѧȡ������ɫ���壬�����Թ��У�����������ϡ���ᣬ�ȣ� | ��ɫ������ȫ��ʧ����Һ�� �� �� ɫ�� |
�˺�ɫ����������ͭ���÷�Ӧ�Ļ�ѧ����ʽΪ CuO+H2SO4�TCuSO4+H2O CuO+H2SO4�TCuSO4+H2O |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�020
���з�Ӧ�����û���Ӧ���ǡ�����
[����]
A.ʵ������п����ϡ����������
B.ʵ�����ü��ȸ�����صķ���������
C.�����ڿ�����ȼ��
D.�ڼ�����������������ԭ����ͭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ�Ͳ��и������п���ѧģ���Ծ��������棩 ���ͣ������
| ʵ�鲽�� | ʵ������ | ���ۺͻ�ѧ����ʽ |
| ����ͬѧȡ������ɫ���壬�����Թ��У�����������ϡ���ᣬ�ȣ� | ��ɫ������ȫ��ʧ����Һ��______ɫ�� | �˺�ɫ����������ͭ���÷�Ӧ�Ļ�ѧ����ʽΪ______ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ɽ��ʡ��̨����Զ���п���ѧģ���Ծ��������棩 ���ͣ������
| ʵ�鲽�� | ʵ������ | ���ۺͻ�ѧ����ʽ |
| ����ͬѧȡ������ɫ���壬�����Թ��У�����������ϡ���ᣬ�ȣ� | ��ɫ������ȫ��ʧ����Һ��______ɫ�� | �˺�ɫ����������ͭ���÷�Ӧ�Ļ�ѧ����ʽΪ______ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com