
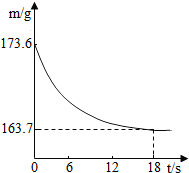
 ij��˾�������Ĵ����Ʒ�����ֻ�����Ȼ������ʣ�Ϊ�ⶨ��Ʒ��̼���Ƶ�����������20��ʱ����ȡ�ò�Ʒ��Ʒ26.5g�����뵽ʢ��һ������ϡ������ձ��У�̼������ϡ����ǡ����ȫ��Ӧ��������ȫ�ݳ����õ��������Ȼ�����Һ����Ӧ�����þ�����������ձ��ڻ�����������m���뷴Ӧʱ�䣨t����ϵ��ͼ��ʾ����
ij��˾�������Ĵ����Ʒ�����ֻ�����Ȼ������ʣ�Ϊ�ⶨ��Ʒ��̼���Ƶ�����������20��ʱ����ȡ�ò�Ʒ��Ʒ26.5g�����뵽ʢ��һ������ϡ������ձ��У�̼������ϡ����ǡ����ȫ��Ӧ��������ȫ�ݳ����õ��������Ȼ�����Һ����Ӧ�����þ�����������ձ��ڻ�����������m���뷴Ӧʱ�䣨t����ϵ��ͼ��ʾ����| 106 |
| x |
| 44 |
| 9.9g |
| 23.85g |
| 26.5g |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����̥��̥ | B��ú�͵Ʒ��� |
| C��ʪ��ұ�� | D��ũ�ҷʵĸ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
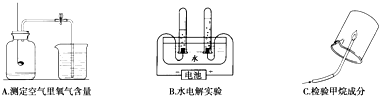
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
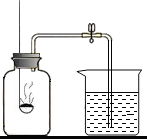 �ڡ����������������IJⶨ��ʵ��̽���У�С����������ͼʵ�飺������ʢ����ȼ�ճ��ڣ���ȼ���������뼯��ƿ�ڲ�����ƿ����
�ڡ����������������IJⶨ��ʵ��̽���У�С����������ͼʵ�飺������ʢ����ȼ�ճ��ڣ���ȼ���������뼯��ƿ�ڲ�����ƿ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
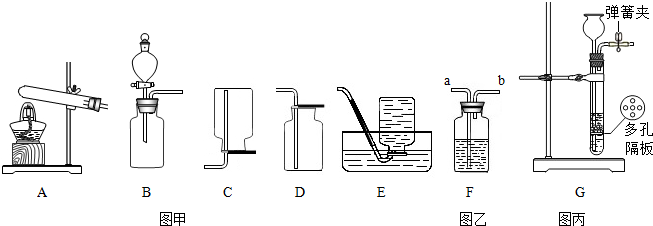
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ѧ�Ҿ��������о�֤������һЩͬѧ���Ե�С��װ��ʳ�У���һ�������ж����к����²��Ļ�ѧ���ʣ���ijЩ��ըʳƷ�к����°����ʱ�ϩ������C3H5ON�������ⶨ��ͼ��Ʒ�к���ϩ������C3H5ON������������Ϊ0.02%��
��ѧ�Ҿ��������о�֤������һЩͬѧ���Ե�С��װ��ʳ�У���һ�������ж����к����²��Ļ�ѧ���ʣ���ijЩ��ըʳƷ�к����°����ʱ�ϩ������C3H5ON�������ⶨ��ͼ��Ʒ�к���ϩ������C3H5ON������������Ϊ0.02%���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com