
| A�� | ��������̼���⡢������Ԫ�ص�������Ϊ3��6��6 | |
| B�� | ����������3��̼ԭ�ӡ�6����ԭ�ӡ�6����ԭ�ӹ��� | |
| C�� | �����ʵ���Է�������Ϊ126g | |
| D�� | �������к�����ԼΪ66.7% |
���� A������Ԫ�������ȵļ��㷽�����ǣ�
B���������ʵĽṹ��������
C��������Է��������ĵ�λ��������
D�����ݻ�������Ԫ�����������ļ��㷽����������
��� �⣺A�������谷��̼���⡢��Ԫ�ص��������ǣ���12��3������1��6������14��6��=6��1��14���ʴ���
B�������谷���ɷ��ӹ��ɵģ���������ԭ��ֱ�ӹ��ɵģ��ʴ���
C����Է��������ĵ�λ���ǡ�g�����ǡ�1����ͨ��ʡ�Բ�д���ʴ���
D�������谷�е�Ԫ�ص�����������$\frac{14��6}{12��3+14��6+1��6}$��100%��66.7%������ȷ��
��ѡD��
���� ������Ҫ����ѧ����Ԫ�������ȵļ��㡢��Է��������ĸ���������Լ�������ɵ��ۺ����㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� | B�� | ���� | C�� | ��ˮ | D�� | ��Ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����CO��NH2��2 | B�� | �����KNO3 | ||
| C�� | ��������Ca��H2PO4��2 | D�� | ��ľ����Ҫ�ɷ�ΪK2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ϡ��Ũ����ʱ����ˮ���ձ�����������Ũ�����У����ò��������Ͻ��� | |
| B�� | ��װ��Ũ������Լ�ƿ���ɿ������� | |
| C�� | ��������ƽ��ȡ 5.4g�Ȼ��� | |
| D�� | ����ҺpHֵʱ��������Һ�㵹����ֽ�ϣ���pH��ֽ����ɫ�����ɫ���Ƚϼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | �� �� | �������� | �����ʵķ��� |
| A | CaCl2��Һ | ϡ���� | �������̼��Ʒ�ĩ������ |
| B | ������̼ | ���� | ͨ�����ȵ�ͭ�� |
| C | CuO���� | Fe3O4 | ������ |
| D | NaCl��Һ | CaCl2 | �������̼������Һ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
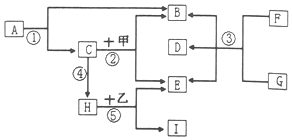 A��I�ͼס�����Ŀǰ������ѧ�ij������ʣ����ǵ��ת����ͼ��ʾ���еķ�Ӧ�����ͷ�Ӧ��δ������������A��B�����Ԫ����ͬ���ҳ����¶���Һ�壮C��ֲ�������õ�һ�ֲ��HΪһ�����壬I�Ǻ�ɫ���壬����ʵ������ȡE�ķ�Ӧ��
A��I�ͼס�����Ŀǰ������ѧ�ij������ʣ����ǵ��ת����ͼ��ʾ���еķ�Ӧ�����ͷ�Ӧ��δ������������A��B�����Ԫ����ͬ���ҳ����¶���Һ�壮C��ֲ�������õ�һ�ֲ��HΪһ�����壬I�Ǻ�ɫ���壬����ʵ������ȡE�ķ�Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ��ʵ | ���� |
| A | ���Ƿŵ�ˮ�������ʧ�� | ���Ƿ��Ӻ�С���������˶� |
| B | ���ʯ��ʯī�������ʲ���ϴ� | ̼ԭ�ӵ����з�ʽ��ͬ |
| C | ��ʯѤ����� | ��ʯ�к���ijЩ����ԭ�� |
| D | ��CO�ж�����CO2�� | ���ӵĽṹ��ͬ |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com