



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢ÓĆŗģĮ׳żČ„ĆܱÕČŻĘ÷ÄŚµÄŃõĘų£ŗ4P+5O2ØT2P2O5 |
| B”¢ÓĆĻ”ŃĪĖį³żĢśŠā£ŗ2HCl+FeOØTFeCl2+H2O |
| C”¢ÓƶžŃõ»ÆĢ¼ÖĘĢ¼ĖįŅūĮĻ£ŗ2CO2”ü+H2OØTH2CO3 |
| D”¢ÓĆ³ĪĒåŹÆ»ŅĖ®¼ģŃéĒāŃõ»ÆÄĘŅѱäÖŹ£ŗNa2CO3+Ca£ØOH£©2ØTCaCO3”ż+2NaOH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
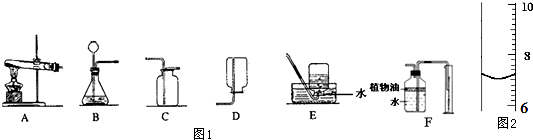
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
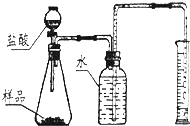 ijŠĖȤŠ”×é°ŃŅ»æé¹ĢĢåĒāŃõ»ÆÄĘ·ÅŌŚ±ķĆęĆóĄļ£¬³¤Ź±¼ä±©Ā¶ŌŚæÕĘųÖŠ£¬·¢ĻÖ¹ĢĢå±ķĆęÖš½„ŹŖČ󣬲æ·ÖČܻƳÉŅŗĢåŗóÓÖÖš½„ŠĪ³É¾§Ģ壬×īÖÕ±ä³É·ŪÄ©£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ijŠĖȤŠ”×é°ŃŅ»æé¹ĢĢåĒāŃõ»ÆÄĘ·ÅŌŚ±ķĆęĆóĄļ£¬³¤Ź±¼ä±©Ā¶ŌŚæÕĘųÖŠ£¬·¢ĻÖ¹ĢĢå±ķĆęÖš½„ŹŖČ󣬲æ·ÖČܻƳÉŅŗĢåŗóÓÖÖš½„ŠĪ³É¾§Ģ壬×īÖÕ±ä³É·ŪÄ©£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
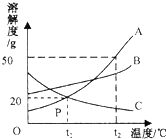 ČēĶ¼ŹĒA”¢B”¢CČżÖÖ¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻߣ®
ČēĶ¼ŹĒA”¢B”¢CČżÖÖ¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻߣ®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
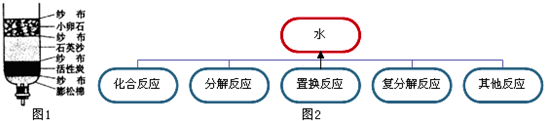
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 Č«ĒņÓµÓŠµÄ»ÆŗĻĪļŅŃ³¬¹ż3000ĶņÖÖ£¬ĘäÖŠ²æ·ÖĪļÖŹŹĒÓÉĒā”¢Ńõ”¢Įņ”¢ÄĘÖŠµÄijŠ©ŌŖĖŲ×é³É£®ĒėÓĆÉĻŹö4ÖÖŌŖĖŲ£¬°“ŅŖĒóÓĆŌŖĖŲ·ūŗÅ»ņ»ÆѧŹ½ĢīæÕ£ŗ
Č«ĒņÓµÓŠµÄ»ÆŗĻĪļŅŃ³¬¹ż3000ĶņÖÖ£¬ĘäÖŠ²æ·ÖĪļÖŹŹĒÓÉĒā”¢Ńõ”¢Įņ”¢ÄĘÖŠµÄijŠ©ŌŖĖŲ×é³É£®ĒėÓĆÉĻŹö4ÖÖŌŖĖŲ£¬°“ŅŖĒóÓĆŌŖĖŲ·ūŗÅ»ņ»ÆѧŹ½ĢīæÕ£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com