
ʵ������һƿ���ʵı�ǩ�����䣬ֻ֪������NH4Cl��(NH4)2SO4��NH4HCO3������[CO(NH2)2]�е�һ�֡������������̽����
�����롿����٣��û�����NH4Cl�� ����ڣ��û�����(NH4)2SO4
����ۣ��û�����NH4HCO3�� ����ܣ��û��������ء�
��ʵ��̽����

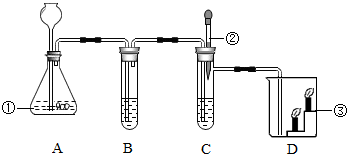
��1��ȡ������Ʒ���в�������ʯ����ĥ�����ݼ�����ζ������ų���֤������ ��������д��������һ���뷢����Ӧ�Ļ�ѧ����ʽ�� ____________________________��
��2�����ϣ�ͼ1������ȡ������Ʒ���Թ��У��μ�����ˮ����ѡ��ͼ2���� ��Һ�����Թ��У��������ݷų�������� ��������
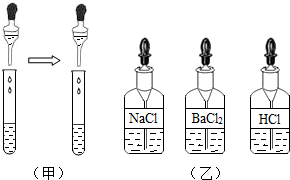
��3���ڲ��裨2�����Թ����ٵ��루ͼ2���е� ____��Һ���� _____________�������ٳ������� ____________________�������ڳ������䷴Ӧ�Ļ�ѧ����ʽ ______________________________________ ��
 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ������һƿ���ʵı�ǩ�����䣬ֻ֪������NH4Cl����NH4��2SO4��NH4HCO3������[CO��NH2��2]�е�һ�֣������������̽����
ʵ������һƿ���ʵı�ǩ�����䣬ֻ֪������NH4Cl����NH4��2SO4��NH4HCO3������[CO��NH2��2]�е�һ�֣������������̽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ������һƿ���ʵı�ǩ�����䣬ֻ֪������NH4Cl����NH4��2SO4��NH4HCO3������[CO��NH2��2]�е�һ�֣������������̽����
ʵ������һƿ���ʵı�ǩ�����䣬ֻ֪������NH4Cl����NH4��2SO4��NH4HCO3������[CO��NH2��2]�е�һ�֣������������̽�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com