
ÓĆĶ¼Ź¾×°ÖĆ²ā¶ØŅņ“ę·Å²»µ±¶ų²æ·Ö±ä³ÉĢ¼ĖįÄʵÄÉÕ¼īÖŠĒāŃõ»ÆÄʵÄÖŹĮæ·ÖŹż”£ĖłČ”ŹŌŃłÖŹĮæ8.00 g”¢×¶ŠĪĘæÖŹĮæ140.00 g£¬¼ÓČė×ćĮæĻ”ĮņĖį(ÖŹĮæĪŖ50.00 g)ĆæøōĻąĶ¬Ź±¼ä¶ĮŹżŅ»“Ī£¬Źż¾ŻČēĻĀ±ķ£ŗ

| ¶ĮŹż“ĪŹż | ÖŹĮæ/g | |
| ׶ŠĪĘæ+ŹŌŃł+Ļ”ĮņĖį | µŚ1“Ī | 197.64 |
| µŚ2“Ī | 197.48 | |
| µŚ3“Ī | 197.26 | |
| µŚ4“Ī | 197.16 | |
| µŚ5“Ī | 197.12 | |
| µŚ6“Ī | 197.12 |
(1)²»±Ų½ųŠŠµŚĘß“Ī¶ĮŹżµÄŌŅņŹĒ___________________________________”£
(2)¼ĘĖćĒāŃõ»ÆÄʵÄÖŹĮæ·ÖŹż”£
(3)ĖłÓĆĻ”ĮņĖįÖŠH2SO4µÄÖŹĮæ·ÖŹż²»µĶÓŚ¶ąÉŁ£æ
½āĪö£ŗ(1)µŚ5“ĪŗĶµŚ6“Ī¶ĮŹżĻąĶ¬£¬ĖµĆ÷ŅŃ³ä·Ö·“Ó¦”£
(2)CO2µÄÖŹĮæm(CO2)=(8.00 g+140.00 g+50.00 g)-197.12 g=0.88 g
ÉčĖłŠčѳʷ֊Na2CO3µÄÖŹĮæĪŖx”£
Na2CO3+H2SO4====H2O+CO2ӟ+Na2SO4
106 44
x 0.88 g
x=(0.88”Į106)/44=2.12 g
ŌņĖłČ”ѳʷ֊NaOHµÄÖŹĮæ·ÖŹżĪŖ£ŗ(5.88/9.00)”Į100%=73.5%
(3)Éčѳʷ֊Na2CO3ĻūŗÄH2SO4µÄÖŹĮæĪŖy”£
Na2CO3+H2SO4====H2O+CO2ӟ+Na2SO4
106 98
2.12 g y
y=(2.12 g”Į98)/106=1.96 g
ŌŁÉčŹŌŃłÖŠNaOHĻūŗÄH2SO4µÄÖŹĮæĪŖz”£
2NaOH+H2SO4====2H2O+Na2SO4
80 98
5.88 z
z=(5.88 g”Į98)/80=7.20 g
ŌņĻ”ĮņĖįÖŠH2SO4µÄÖŹĮæ·ÖŹż²»µĶÓŚ
£Ū(1.96 g+7.20 g)/50.00 g£Ż”Į100%=18.32%
“š°ø£ŗ(1)µŚ5”¢6“Ī¶ĮŹżĻąĶ¬ (2)73.5% (3)18.32%


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ÓĆĶ¼Ź¾×°ÖĆ²ā¶ØŅņ“ę·Å²»µ±¶ų²æ·Ö±ä³ÉĢ¼ĖįÄʵÄÉÕ¼īÖŠĒāŃõ»ÆÄʵÄÖŹĮæ·ÖŹż£®ĖłČ”ŹŌŃłÖŹĮæ8.00 g”¢×¶ŠĪĘæÖŹĮæ140.00 g£¬¼ÓČė×ćĮæĻ”ĮņĖį£ØÖŹĮæĪŖ50.00 g£©ĆæøōĻąĶ¬Ź±¼ä¶ĮŹżŅ»“Ī£¬Źż¾ŻČēĻĀ±ķ£ŗ
ÓĆĶ¼Ź¾×°ÖĆ²ā¶ØŅņ“ę·Å²»µ±¶ų²æ·Ö±ä³ÉĢ¼ĖįÄʵÄÉÕ¼īÖŠĒāŃõ»ÆÄʵÄÖŹĮæ·ÖŹż£®ĖłČ”ŹŌŃłÖŹĮæ8.00 g”¢×¶ŠĪĘæÖŹĮæ140.00 g£¬¼ÓČė×ćĮæĻ”ĮņĖį£ØÖŹĮæĪŖ50.00 g£©ĆæøōĻąĶ¬Ź±¼ä¶ĮŹżŅ»“Ī£¬Źż¾ŻČēĻĀ±ķ£ŗ| ¶ĮŹż“ĪŹż | ÖŹĮæ/g | |
| ׶ŠĪĘæ+ŹŌŃł+Ļ”ĮņĖį | µŚ1“Ī | 197.64 |
| µŚ2“Ī | 197.48 | |
| µŚ3“Ī | 197.26 | |
| µŚ4“Ī | 197.16 | |
| µŚ5“Ī | 197.12 | |
| µŚ6“Ī | 197.12 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆĶ¼Ź¾×°ÖĆ²ā¶ØŅņ“ę·Å²»µ±¶ų²æ·Ö±ä³ÉĢ¼ĖįÄʵÄÉÕ¼īÖŠĒāŃõ»ÆÄʵÄÖŹĮæ·ÖŹż”£ĖłČ”ŹŌŃłÖŹĮæ8.00 g”¢×¶ŠĪĘæÖŹĮæ140.00 g£¬¼ÓČė×ćĮæĻ”ĮņĖį(ÖŹĮæĪŖ50.00 g)ĆæøōĻąĶ¬Ź±¼ä¶ĮŹżŅ»“Ī£¬Źż¾ŻČēĻĀ±ķ£ŗ
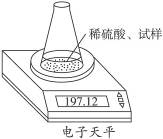
| ¶ĮŹż“ĪŹż | ÖŹĮæ/g | |
| ׶ŠĪĘæ+ŹŌŃł+Ļ”ĮņĖį | µŚ1“Ī | 197.64 |
| µŚ2“Ī | 197.48 | |
| µŚ3“Ī | 197.26 | |
| µŚ4“Ī | 197.16 | |
| µŚ5“Ī | 197.12 | |
| µŚ6“Ī | 197.12 |
(1)²»±Ų½ųŠŠµŚĘß“Ī¶ĮŹżµÄŌŅņŹĒ___________________________________”£
(2)¼ĘĖćĒāŃõ»ÆÄʵÄÖŹĮæ·ÖŹż”£
(3)ĖłÓĆĻ”ĮņĖįÖŠH2SO4µÄÖŹĮæ·ÖŹż²»µĶÓŚ¶ąÉŁ£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
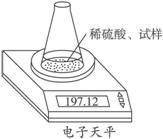
ÓĆĶ¼Ź¾×°ÖĆ²ā¶ØŅņ“ę·Å²»µ±¶ų²æ·Ö±ä³ÉĢ¼ĖįÄʵÄÉÕ¼īÖŠĒāŃõ»ÆÄʵÄÖŹĮæ·ÖŹż”£ĖłČ”ŹŌŃłÖŹĮæ8.00 g”¢×¶ŠĪĘæÖŹĮæ140.00 g£¬¼ÓČė×ćĮæĻ”ĮņĖį(ÖŹĮæĪŖ50.00 g)ĆæøōĻąĶ¬Ź±¼ä¶ĮŹżŅ»“Ī£¬Źż¾ŻČēĻĀ±ķ£ŗ
| ¶ĮŹż“ĪŹż | ÖŹĮæ/g | |
| ׶ŠĪĘæ+ŹŌŃł+Ļ”ĮņĖį | µŚ1“Ī | 197.64 |
| µŚ2“Ī | 197.48 | |
| µŚ3“Ī | 197.26 | |
| µŚ4“Ī | 197.16 | |
| µŚ5“Ī | 197.12 | |
| µŚ6“Ī | 197.12 |
(1)²»±Ų½ųŠŠµŚĘß“Ī¶ĮŹżµÄŌŅņŹĒ___________________________________”£
(2)¼ĘĖćĒāŃõ»ÆÄʵÄÖŹĮæ·ÖŹż”£
(3)ĖłÓĆĻ”ĮņĖįÖŠH2SO4µÄÖŹĮæ·ÖŹż²»µĶÓŚ¶ąÉŁ£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗĶ¬²½Ģā ĢāŠĶ£ŗ¼ĘĖćĢā


²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com