
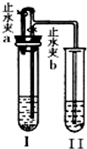
 ij»ÆѧŠ”×éĶ¬Ń§°“ČēĶ¼ĖłŹ¾×°ÖĆŗĶ±ķÖŠĖłøųŹŌ¼Į½ųŠŠŹµŃé£ØĶ¼ÖŠĢś¼ÜĢØµČ¼Š³ÖŅĒĘ÷¾łŅŃĀŌČ„£©£ŗ
ij»ÆѧŠ”×éĶ¬Ń§°“ČēĶ¼ĖłŹ¾×°ÖĆŗĶ±ķÖŠĖłøųŹŌ¼Į½ųŠŠŹµŃé£ØĶ¼ÖŠĢś¼ÜĢØµČ¼Š³ÖŅĒĘ÷¾łŅŃĀŌČ„£©£ŗ| ×鱚 | A×é | B×é | C×é | D×é |
| ŹŌ¹ÜI | Ca£ØOH£©2 Ļ”HCl |
CaCO3 Ļ”HCl |
Zn Ļ”H2SO4 |
Cu Ļ”H2SO4 |
| ŹŌ¹Ü¢ņ | KNO3ČÜŅŗ | Ca£ØOH£©2ČÜŅŗ | Ba£ØOH£©2ČÜŅŗ | Ba£ØOH£©2ČÜŅŗ |


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗŗ£ÄĻŹ”¾ŗČüĢā ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
| ×鱚 | A×é | B×é | C×é | D×é |
| ŹŌ¹ÜI | Ca£ØOH£©2 Ļ”HCl |
CaCO3 Ļ”HCl |
Zn Ļ”H2SO4 |
Cu Ļ”H2SO4 |
| ŹŌ¹Ü¢ņ | KNO3ČÜŅŗ | Ca£ØOH£©2ČÜŅŗ | Ba£ØOH£©2ČÜŅŗ | Ba£ØOH£©2ČÜŅŗ |
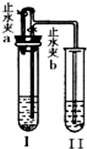
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźµŚ¶žŹ®½ģ”°ĢģŌ±”±Č«¹ś³õ֊ѧɜ»ÆѧĖŲÖŹŗĶŹµŃéÄÜĮ¦¾ŗČü³õČüŹŌ¾ķ£ØĒķŗ£ŹŠŗ£¹šŃ§Š££©£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
| ×鱚 | A×é | B×é | C×é | D×é |
| ŹŌ¹ÜI | Ca£ØOH£©2 Ļ”HCl | CaCO3 Ļ”HCl | Zn Ļ”H2SO4 | Cu Ļ”H2SO4 |
| ŹŌ¹Ü¢ņ | KNO3ČÜŅŗ | Ca£ØOH£©2ČÜŅŗ | Ba£ØOH£©2ČÜŅŗ | Ba£ØOH£©2ČÜŅŗ |
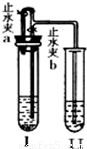
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com