
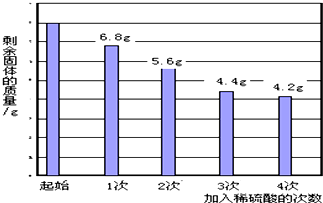
 ����ͭ�����þ������п�е�һ����ɵĻ�����ȤС���ͬѧ���ⶨ����ɣ���������ʵ�飬ȡ�û�����ĩ8.0g�����ձ��У���140.0g ���ʵ���������Ϊ14.0%��ϡ����ƽ�����Ĵμ�����ձ��У���ַ�Ӧ���ʣ������������ݼ�¼��ͼ������ͨ������ش�
����ͭ�����þ������п�е�һ����ɵĻ�����ȤС���ͬѧ���ⶨ����ɣ���������ʵ�飬ȡ�û�����ĩ8.0g�����ձ��У���140.0g ���ʵ���������Ϊ14.0%��ϡ����ƽ�����Ĵμ�����ձ��У���ַ�Ӧ���ʣ������������ݼ�¼��ͼ������ͨ������ش����� þ������п��ϡ���ᷴӦ������������Ӧ���Σ�����ͼ���ṩ�����ݿ��Խ�����ط���ļ�����жϣ�
��� �⣺��1��������ķ�����M�����ԭ������Ϊx��
��һ�η�Ӧ�Ľ�������Ϊ��8.0g-6.8g=1.2g����Ӧ����������Ϊ��35.0g��14.0%=4.9g��
M+H2SO4�TMSO4+H2����
x 98
1.2g 4.9g
$\frac{x}{1.2g}$=$\frac{98}{4.9g}$��
x=24��
������þ����˻������ͭ��þ�Ļ���
�û�����ĩ��ͭ����������Ϊ��$\frac{4.2g}{8.0g}$��100%=52.5%��
��ͭ������������52.5%��
��2��������μ��������ַ�Ӧ����������þ����Ϊy��������������Ϊz��
Mg+H2SO4�TMgSO4+H2����
24 120 2
8g-4.4g y z
$\frac{24}{8g-4.4g}$=$\frac{120}{y}$=$\frac{2}{z}$��
y=18g��z=0.3g��
������Һ�����ʵ���������Ϊ��$\frac{18g}{3.6g+105g-0.3g}$��100%=16.6%��
��������Һ�����ʵ���������Ϊ16.6%��
���� ������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ | B�� | ��ԭ�� | C�� | �ȶ��� | D�� | ��ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
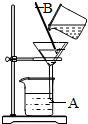 ��ͼ��ʵ�����й��˲���ʾ��ͼ���ش��������⣺
��ͼ��ʵ�����й��˲���ʾ��ͼ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ܢۢ٢ڢ� | B�� | �ڢ٢ܢۢ� | C�� | �ڢܢ٢ۢ� | D�� | �ܢ٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com