
 ×100%���㼴�ɣ�
×100%���㼴�ɣ�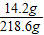 ×100%=6.5%��
×100%=6.5%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�013
(2004
�����)������Ȫˮ��Դ�ḻ�������ǣ����С�Ȫ�ǡ�������2003����ͻȪ�ĸ�ӿ��Ȫ�Ǵ����˲�������������ˮ��Ȫ���Ż���������ֹ��Ⱦ���ѳ�ΪȪ���˵Ĺ�ʶ��������١������У�����Ϊ�����л����ĸ��Ʋ��������[
����]�����ⶪ���Ͼɵ��
��������ˮ���������
�۽������������뻤�Ǻ��ڻ�͵ط���
�ܹ�ҵ��ˮ��������ѭ��ʹ��
|
A ��ֻ�Т٢� |
B ��ֻ�Тڢ� |
|
C ��ֻ�Т٢� |
D ��ֻ�Т٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009���Ĵ�ʡ�ɶ����������п���ѧģ���Ծ��������������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2004��ɽ��ʡ�������п���ѧ�Ծ��������棩 ���ͣ������
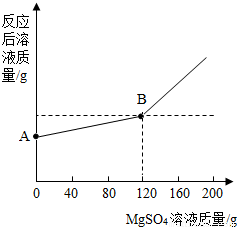
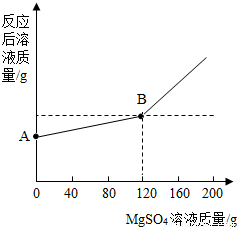
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2004��ɽ��ʡ�������п���ѧ�Ծ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com