
������̼�ġ������롰��桱��ʵ������������ŵ���Ҫ;��֮һ��ʵ�������У�������������NaOH��Һ��������CO2������ͼ����(��������������δ���)��
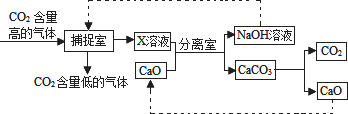
(1)�������н��еIJ�����________��
(2)������ͼ������������У�����ˮ�ų������ȵ���������________��
(3)�����ҡ��ڷ�����Ӧ�Ļ�ѧ����ʽΪ________��
(4)��CaO���뵽x��Һ�У����з������ֽⷴӦ�Ļ�ѧ����ʽ��________��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
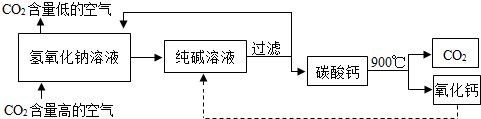
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
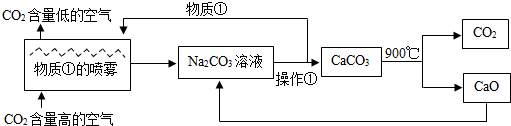
| A���÷����а������ֽⷴӦ���ֽⷴӦ���û���Ӧ�� | B�����ʢ���Ca��OH��2��Һ | C���������ǹ��� | D����������������NaOH��CO2�������ʿ���ѭ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com