
��Ҫ��д����ѧ����ʽ���ش��й����⣺
��1��ˮ��ͨ��ʱ������⣺��_ ________����
�ڸ�ʵ���У��ɿ��������븺������������������_________������ʵ��ó��Ľ�����ˮ����____ _____��ɡ�
��2��ϸ��˿�ڴ�������ȼ�գ���__ _______����
��ʵ����Ҫ����ƿ��Ԥ�ȷ�������ˮ����ϸɳ��ԭ������___ __ ____ ����
��3����ͼ��ͼ�к���ȼ�յĻ�ѧ����ʽΪ��_______ __ ����
��ֹˮ�к�����������_______ __��˵��������������Լռ_ ___������ͨ��ʵ��ó�������۵Ļ�ѧ����__ __������ţ���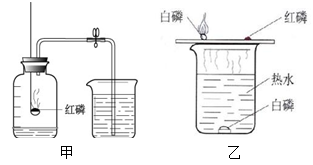
| A�������� | B�������� | C�������� | D����ķ�� |
��1��2H2O 2H2��+ O2 �� 1��2 ��Ԫ�غ���Ԫ��
2H2��+ O2 �� 1��2 ��Ԫ�غ���Ԫ��
��2��3Fe + 2O2 Fe3O4 ��ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը��
Fe3O4 ��ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը��
��3��4P + 5O2 2P2O5 ˮ����ƿ�У���Լռƿ�������������1/5 1/5 A
2P2O5 ˮ����ƿ�У���Լռƿ�������������1/5 1/5 A
���������������1�����ˮʵ�飬���Դ������������������ʹ�����ǵ�ľ����ȼ�������������Դ��������������������ȼ�գ���������ɫ�Ļ��桪�����������Է���ʽ��2H2O 2H2��+ O2 �������ˮ�ھ����������⣬�����һ�����������غ㶨�ɣ�Ԫ�ص������ڷ�Ӧǰ�䣬���Խ����ǣ�ˮ����Ԫ�غ���Ԫ�����
2H2��+ O2 �������ˮ�ھ����������⣬�����һ�����������غ㶨�ɣ�Ԫ�ص������ڷ�Ӧǰ�䣬���Խ����ǣ�ˮ����Ԫ�غ���Ԫ�����
��2����˿ȼ�յķ���ʽ��3Fe + 2O2 Fe3O4��Ϊ�˷�ֹ��ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը�ѣ�Ҫ����ƿ��Ԥ�ȷ�������ˮ����ϸɳ
Fe3O4��Ϊ�˷�ֹ��ֹ���ɵĹ������ʽ���ƿ�ף���ʹ����ƿը�ѣ�Ҫ����ƿ��Ԥ�ȷ�������ˮ����ϸɳ
��3������ȼ�յķ���ʽ��4P + 5O2 2P2O5�����ں���ȼ��������ƿ�ڵ�������ʹ��ƿ�ڵ����������٣�ѹǿ���٣�����ˮ����ƿ�У���Լռƿ�������������1/5������ˮ����ƿ�У���Լռƿ�������������1/5��˵�������������ĺ�����Լ��1/5������ͨ��ʵ��ó�������۵Ļ�ѧ���ǣ�����������ѡA
2P2O5�����ں���ȼ��������ƿ�ڵ�������ʹ��ƿ�ڵ����������٣�ѹǿ���٣�����ˮ����ƿ�У���Լռƿ�������������1/5������ˮ����ƿ�У���Լռƿ�������������1/5��˵�������������ĺ�����Լ��1/5������ͨ��ʵ��ó�������۵Ļ�ѧ���ǣ�����������ѡA
���㣺ˮ�ĵ��ʵ�飬��˿ȼ�գ����������������IJⶨ


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣���ѧ�о����ʵ���ɡ��ṹ�����ʡ��仯֮��Ĺ�ϵ��
��1����ѧ��ͨ���о�ˮ�ı仯��ʶ��ˮ����ɡ���ͼΪ���ˮ�ļ���װ�ã��Թܢ��в����������� ���ɴ�ʵ��ó�ˮ���� ��ɵģ�2����ɺ������������е���ϵ������Һ�������ƵĻ�ѧ��������Ϊ���ж����� ��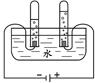
��3���ṹ�������ʡ��о����ֺ��С�������������O��O���������ʾ��к�ǿ�������ԣ�������Ϊɱ�����������ݴ��Ʋ⣬���������У�������ɱ������������ (���������)��
��4��ͨ��������ɺͽṹ�����ǿ���Ԥ�����ʵ�ijЩ���ʡ�����NaHSO4���ʵ������Ʋ⣬�������� ����������ţ���
����ˮ��Һ����ط����û���Ӧ���õ�������
����ˮ��Һ��ʹ��ɫʯ����Һ���
����ˮ��Һ����п��Ӧ��������
����ˮ��Һ�������ᱵ��Ӧ�������ᱵ����������ʾ�����ᱵ������ˮ��Ҳ�������ᣩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧΪ�ⶨ�����������ĺ������������ͼ��ʾ��ʵ��װ�á���ͬѧ�ڡ������ݡ���ÿһ���İ�������һ������ֽ����ˮ��İ��ף��÷Ŵ��6V�ֵ�Ͳ���ڿ���ˮ���һ���������ݡ����İ����ϡ�
��1��һ��ʱ��ɹ۲쵽����Ҫ������ ��
��2���������ݡ���ÿһ���϶�����һС�Ű�����ֻ����ˮ���һ���������ݡ�����һ��Ű�����ȣ��ŵ��� ��
��3��д������ѧ���IJ�����ʵ������ķ�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ijͬѧ��ʵ���Ҽ��ȸ��������ȡ������װ��ͼ��
��ش��������⣺
��1����ͼ�б������A�����ƣ�
��2����ʵ��װ��ͼ����������
��3��д����ȡ�����Ļ�ѧ����ʽ ��
��4������A�ڸ�ʵ���е������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ���Ǻ�ˮ���ܽ���η��и������ӵ������������������ͼʾ�ش��������⣺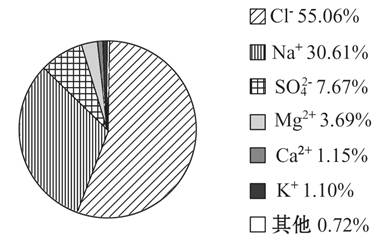
(1)��ˮ�к������Ľ��������� (�����ӷ���)��
(2)þ���ӵĺ���Ϊ ��
(3)���к������ķǽ������Ӻͺ����ڶ���Ľ��������γɵĻ�����Ļ�ѧʽ�� ��
(4)ͼ�и������������ӿ����γ� ���Σ������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ϣ����ʮ�������������ϵ����ʲ��ϵر仯�������ijɷ�Ҳ�����˺ܴ�ı仯���±���ԭʼ������Ŀǰ��������Ҫ�ɷ֣�
| Ŀǰ��������Ҫ�ɷ� | N2��O2��CO2��ˮ��������������� |
| ԭʼ��������Ҫ�ɷ� | CH4��NH3��CO��CO2�� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ɶ���������ɵĻ�����һ�ֱ������Ȼ��Դ��
��1��ʵ���ҳ��ú���ȼ�����ⶨ�����������ĺ������÷�Ӧ�Ļ�ѧ����ʽΪ�� ��ʵ����ֲ�õ������������С��1/5��������ֽ���Ŀ���ԭ���� ����һ��ԭ��
��2��������ϡ����������������ԼΪ ,ͨ�������� ��
��3��2013��6��12�գ�����ʮ�ź���Ա��̫���жȹ����й���ͳ���ա�������ڣ����ҳԵ������ӡ����Ӳ�����հ�װ��Ϊ�� ��
��4����ͬ���������ܶ����ǽ������绷���յ��й����⡣������PM2��5������ᵼ�����������������������º������������࣬��PM2��5ר�ÿ��������˻���̿�� ���á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ˮ�Ͷ�����̼������������Ҫ�����ʡ�
��1��A��B��C�����о�������ɺ����ʵ�ʵ�顣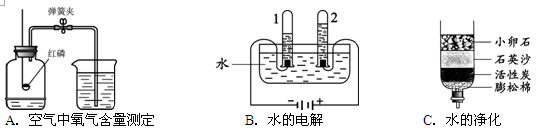
�ٹ���Aͼ��ʾʵ�飬����˵������ȷ���� ��
A��ʵ��ʱ����Ӧ���� B����ȼ����ǰ���õ��ɼмн��齺��
C������Ϩ������̴��ɼ� D�����ս���ƿ��ˮ�����ԼΪ���������
��Bͼ�Թ�1�е�����Ϊ ��Cͼ�о���ˮ�ķ����� ��������
��2����ʯ�ҽ�Ĩǽ��һ��ʱ���ǽ�ڱ���ְ���Ӳ��ԭ���� ���û�ѧ����ʽ�ش𣩡�
��3��������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ�¶�����̼�Ͱ�����NH3�����Ժϳ�����[CO(NH2)2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����̼�����Ե��ܺġ����ŷš�����ȾΪ��������ʵ���������Դ����Ч�ʺʹ��������Դ�ṹ������˵���У���ȷ���� ��
A����������ʱ����ú������ú����ʹ��
B����У��������͵��������շ��糧�����շ���
C���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ�
D������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դx
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2012��3��22���ǵڶ�ʮ�조����ˮ�ա���ˮ����������������������أ�
��1����Լ��ˮ����ֹˮ��ȾӦ��Ϊ���ǵ��Ծ���Ϊ�������й������в���ȷ������ ��
A���������������õ���ˮϰ�ߣ������ܳ������ÿһ��ˮ
B����ҵ��ˮ���������������ŷ�
C��ˮ����Ⱦ��Σ�����彡��
D������ʹ��ũҩ�����ʣ��������ˮ����Ⱦ
��2��ˮ��������ܼ����������������ʷֱ����ˮ�У������γ���Һ������ ����
A��ֲ���� B������ C������ D���������
��3��ˮ�ľ����������� �������������ˡ�����
��4�����з�Ӧ�У���������ȷ��ˮ���⡢��Ԫ����ɵ����� ����
A��H2��O2��Ӧ B�����ˮ
C��H2��Cl2��Ӧ D��H2��CuO��Ӧ����ʾ��CuO+H2 Cu+H2O��
Cu+H2O��
��5����ͼ��ʾ��ʵ��1���Ʊ�����ˮ��װ�ã�ʵ��2�ǵ��ˮ��װ�ã�
��ʵ��I��ˮ��A��Bת�Ƶ�C�Ĺ����У�ˮ���ӵ����û�з����仯�������仯����ˮ���ӵ��� ����
��ʵ��II�з�Ӧ�Ļ�ѧ����ʽΪ�� ����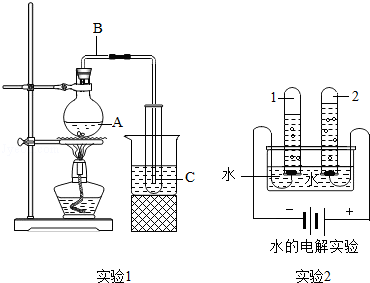
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com