�������ƣ�NaN
3�����㷺Ӧ����������ȫ���ң�ij��ȤС�������������о���
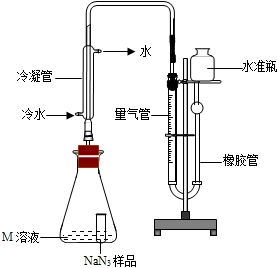
���������ϡ�
��1��������ײ����30����������NaN
3��Ѹ�ٷֽ�ΪNa��N
2��
��2�������ɵĽ�������Һ̬����Ӧ��NaNH
2���ٽ�NaNH
2��N
2O��Ӧ������NaN
3��NaOH������X���÷�Ӧ�Ļ�ѧ����ʽΪ2NaNH
2+N
2O=NaN
3+NaOH+X����Ϊ
NH3
NH3
��ʵ���Ҽ��������ʹ�õ���ֽ��ʪ��
��ɫʯ����ֽ
��ɫʯ����ֽ
�������Ʊ���Ӧ����ҵ��NaN
3�лẬ��Na
2CO
3���Է�����ҵ��NaN
3���Na
2CO
3�Ŀ���ԭ��
����NaN2��ͬʱ��NaOH���ɣ�NaOH���տ����е�CO2����Na2CO3
����NaN2��ͬʱ��NaOH���ɣ�NaOH���տ����е�CO2����Na2CO3
��
��Na
2CO
3���������ⶨ��
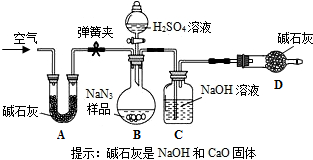
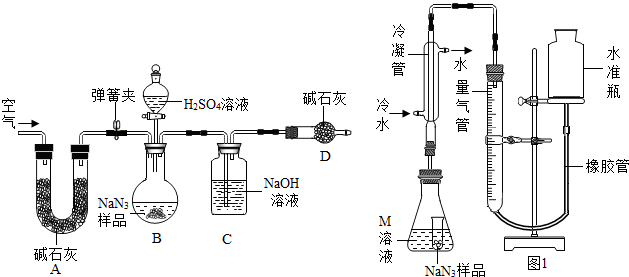
Ϊ��һ���ⶨNaN
3��Ʒ��Na
2CO
3�����������������ͼװ�ã���֪H
2SO
4��Һ��NaN
3����Ӧ���������壩��
ʵ�鲽�����£�
�ٰ�ͼ����װ�ã�
���װ�õ�������
���װ�õ�������
��
��ȷ�Ƶ�ʢ�м�ʯ�ң������������ƺ������ƵĻ����ĸ����D������Ϊa g��
��ȷ�Ƶõ������ƣ�NaN
3����Ʒ������W g����װ��B�Ĺ��ƿ�У�
�ܴ�װ��B�ķ�Һ©����������������ϡ���ᣬ�����ٲ�������Ϊֹ��
�ݴ��ɼУ����Թ�A�л���������������ӣ�Ȼ���ٳƵø����D��������Ϊb g������ʵ���в�õ��й����ݣ����������Ʒ��̼���Ƶ�����������
�Իش𣺣�1���ڢݲ��������������Ŀ����
��B�����ɵĶ�����̼����D��
��B�����ɵĶ�����̼����D��
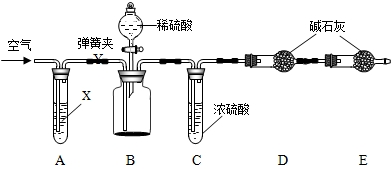
��װ��A���Լ�X������ѡ��
����������Һ
����������Һ
��д��B����������ķ�Ӧ����ʽ
Na2CO3+H2SO4=Na2SO4+H2O+CO2��
Na2CO3+H2SO4=Na2SO4+H2O+CO2��
��
��2����û��Cװ�ã���ᵼ�²ⶨ���
ƫ��
ƫ��
�����ƫ��ƫС������
��3��Eװ�õ�������
���տ����е�ˮ�Ͷ�����̼
���տ����е�ˮ�Ͷ�����̼
��




 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�