
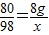



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2008��ɽ��ʡ�����г��б�ҵ��ѧͳһ���ԡ���ѧ�Ծ� ���ͣ�043
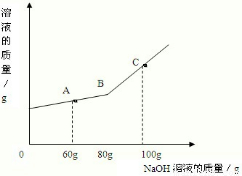
��һ�ձ���ʢ��һ��������MgCO3���壬�����еμ����ʵ��ʾ�����Ϊ10����H2SO4��Һ����ǡ����ȫ��Ӧ���õ�102 g��������Һ����������Һ����ε���������������Ϊl0����NaOH��Һ������������������������NaOH��Һ��������ϵ������ͼ��ʾ�����������ش��������⣺
(1)�ڵ���ϡ����ʱ���۲쵽������ʵ��������________��
(2)������NaOH��Һ��ͼ��A��ʱ���ձ�����Һ�ﺬ�е�������(д��ѧʽ)________��
(3)������10����NaOH��Һ80 gʱ(��B��)����ͨ�����㣬���ʱ����С������Һ��������(��������ȷ��0.1 g)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�п����� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�ձ���ʢ��һ��������MgCO3���壬�����еμ����ʵ��ʾ�����Ϊ10����H2SO4��Һ����ǡ����ȫ��Ӧ���õ�102g��������Һ����������Һ����ε���������������Ϊl0����NaOH��Һ������������������������NaOH��Һ��������ϵ������ͼ��ʾ�����������ش��������⣺
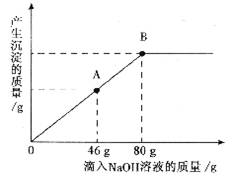
��1���ڵ���ϡ����ʱ���۲쵽������ʵ��������______________________________��
��2��������NaOH��Һ��ͼ��A��ʱ���ձ�����Һ�ﺬ�е������ǣ�д��ѧʽ�� ____________________________________________________________________________��
��3��������10����NaOH��Һ80gʱ����B�㣩����ͨ�����㣬���ʱ����С������Һ������������������ȷ��0.1g��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com