
·ÖĪö ±¾Ģāæ¼²é»ÆѧÓĆÓļµÄŅāŅå¼°ŹéŠ“£¬½āĢā¹Ų¼üŹĒ·ÖĒå»ÆѧÓĆÓļĖł±ķ“ļµÄ¶ŌĻóŹĒ·Ö×Ó”¢Ō×Ó”¢Ąė×Ó»¹ŹĒ»ÆŗĻ¼Ū£¬²ÅÄÜŌŚ»Æѧ·ūŗÅĒ°»ņĘäĖüĪ»ÖĆ¼ÓÉĻŹŹµ±µÄ¼ĘĮæŹżĄ“ĶźÕūµŲ±ķ“ļĘäŅāŅ壬²¢ÄÜøł¾ŻĪļÖŹ»ÆѧŹ½µÄŹéŠ“¹ęŌņÕżČ·ŹéŠ“ĪļÖŹµÄ»ÆѧŹ½£¬²ÅÄÜŹģĮ·×¼Č·µÄ½ā“š“ĖĄąĢāÄ森
½ā“š ½ā£ŗ£Ø1£©¢Ł³£ÓĆÓŚÖŠŗĶĖįŠŌĶĮČĄµÄ¼īŹĒĒāŃõ»ÆøĘ£¬øĘŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ+2£¬ĒāŃõøłµÄ»ÆŗĻ¼ŪĪŖ-1£¬ĒāŃõ»ÆøʵĻÆѧŹ½ĪŖ£ŗCa£ØOH£©2£»
¢ŚĢģČ»ĘųÖ÷ŅŖ³É·ÖŹĒ¼×Ķ飬æÉŅŌ±ķŹ¾ĪŖCH4£»
¢ŪĪ¶Ę·Ź³“×ÖŠŗ¬ÓŠµÄĖįŹĒ“×Ėį£¬Ęä»ÆѧŹ½ĪŖ£ŗCH3COOH£®
¢ÜŗīŹĻÖĘ¼ī·ØÖŠµÄ”°¼ī”±ŹĒĢ¼ĖįÄĘ£¬»ÆѧŹ½ĪŖ£ŗNa2CO3£®
¢ŻŹµŃéŹŅ×ī³£ÓƵÄČ¼ĮĻŹĒ¾Ę¾«£¬Ęä»ÆѧŹ½ĪŖ£ŗC2H5OH£®
¢ŽŃõ»ÆøĘÄÜÓėĖ®·“Ӧɜ³ÉĒāŃõ»ÆøĘ£¬ŹĒ³£ÓĆ×öøÉŌļ¼ĮµÄŃõ»ÆĪļ£¬Ęä»ÆѧŹ½ĪŖ£ŗCaO£®
£Ø2£©Ļ“½ą¾«¾ßÓŠČé»Æ¹¦ÄÜ£¬Äܽ«ÓĶĪŪ±ä³ÉĪ¢Š”µÄÓĶÖ¬Š”ŅŗµĪ£¬Ņņ“ĖÄÜøüČŻŅ×Ļ“µō£®
£Ø3£©ĢśŌŚÓėŃõĘųŗĶĖ®³ä·Ö½Ó“„Ź±ČŻŅ×ÉśŠā£®¹ŹĢī£ŗŃõĘų£»Ė®ÕōĘų
£Ø4£©A”¢ÓĆĮæĶ²ĮæČ”Ė®Ź±ø©ŹÓ¶ĮŹż£¬ĖłĮæµÄĖ®Ę«ÉŁ£¬ĖłµĆČÜŅŗÖŠĀČ»ÆÄʵÄÖŹĮæ·ÖŹż»įĘ«“󣻣®²»·ūŗĻŅŖĒ󣬹Ź²»Ń”A£»
B”¢ÓĆĖ®ČóĻ“ÉÕ±Ź±£¬ÉÕ±±Ś²ŠĮōµÄĖ®Ź¹ĖłµĆČÜŅŗÖŠČܼĮĖ®µÄĮæĘ«“ó£¬Ōģ³ÉÖŹĮæ·ÖŹżĘ«Š”£®²»·ūŗĻŅŖĒ󣬹Ź²»Ń”B£»
C”¢³ĘČ”µÄĀČ»ÆÄĘ¹ĢĢå²»“棬ÖĀŹ¹ČÜÖŹÖŹĮæŠ”ÓŚÅäÖĘĖłŠčŅŖµÄČÜÖŹÖŹĮ棬ŅżĘšÖŹĮæ·ÖŹżĘ«Š”£®²»·ūŗĻŅŖĒ󣬹Ź²»Ń”C£»
D”¢ŅŃÅäÖĘŗƵÄČÜŅŗ£¬øł¾ŻČÜŅŗµÄ¾łŅ»ŠŌ£¬ČÜÖŹÖŹĮæ·ÖŹż²»»įŅņÉŁĮ潦³ö¶ų·¢Éśøı䣬Ņņ“ĖøĆ²Ł×÷²»»įŅżĘšÖŹĮæ·ÖŹżĘ«Š”£®
¹ŹæÉŃ”BC£»
¹Ź“š°øĪŖ£ŗ£Ø1£©¢ŁCa£ØOH£©2 ¢ŚCH4 ¢ŪCH3COOH ¢ÜNa2CO3 ¢ŻC2H5OH ¢ŽCaO
£Ø2£©·¢ÉśČé»Æ×÷ÓĆ£® £Ø3£©ŃõĘųŗĶĖ® £Ø4£©BC
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éѧɜ¶Ō»ÆѧÓĆÓļµÄŹéŠ“ŗĶĄķ½āÄÜĮ¦£¬ĢāÄæÉč¼Ę¼Č°üŗ¬¶Ō»Æѧ·ūŗÅŅāŅåµÄĮĖ½ā£¬ÓÖæ¼²éĮĖѧɜ¶Ō»Æѧ·ūŗÅµÄŹéŠ“£¬æ¼²éČ«Ćę£¬×¢ÖŲ»ł“”£¬ĢāÄæÄŃ¶Č½ĻŅ×£®


 ÓäæģµÄŗ®¼ŁÄĻ¾©³ö°ęÉēĻµĮŠ“š°ø
ÓäæģµÄŗ®¼ŁÄĻ¾©³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µēĻ߶ĢĀ·Ęš»šÓĆĖ®ĘĆĆš | B£® | ץÉĻ¾Ę¾«×Å»š£¬ÓĆŹŖÄز¼øĒĆš | ||
| C£® | µ±ĻØĆš¾Ę¾«µĘŹ±ÓĆ×ģ“µĆš | D£® | ×öĶźŹµŃéŗóŹ£ÓąŅ©Ę··Å»ŲŹŌ¼ĮĘæÖŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ö²ĪļµÄ¹āŗĻ×÷ÓĆ | |
| B£® | ŅŌŗ£Ė®ÖŠµÄĀČ»ÆÄʵČĪŖŌĮĻÖĘČ”“æ¼ī | |
| C£® | ÉśŹÆ»Ņ±ä³ÉŹģŹÆ»Ņ | |
| D£® | ÅØŃĪĖį³ØæŚ·ÅÖĆæÕĘųÖŠÖŹĮæ¼õĒį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĀČ»ÆĢśČÜŅŗÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦ŗóĮōĻĀµÄŗģŗÖÉ«¹ĢĢå | |
| B£® | ¼īŹ½Ģ¼ĖįĶŹÜČČ·Ö½āŗóµÄŗŚÉ«¹ĢĢå[Cu2£ØOH£©2CO3$\frac{\underline{\;\;”÷\;\;}}{\;}$2CuO+H2O+CO2”ü] | |
| C£® | ÓĆ×ćĮæĒāĘų»¹ŌŃõ»ÆĶŗóĮōĻĀµÄŗģÉ«¹ĢĢå | |
| D£® | Ź¢·ÅŹÆ»ŅĖ®ŗóĮōĻĀµÄ°×É«¹ĢĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 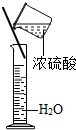 Ļ”ŹĶÅØĮņĖį | B£® |  ĻņŹŌ¹ÜÄŚĒćµ¹ŅŗĢå | ||
| C£® | 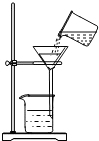 ¹żĀĖ | D£® | 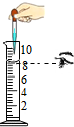 ĮæČ”ŅŗĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| Ō×ÓÖÖĄą | ÖŹ×ÓŹż | ÖŠ×ÓŹż | ŗĖĶāµē×ÓŹż | ŗĖµēŗÉŹż |
| A | ||||
| B | 10 | |||
| C | ||||
| D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com