
�����ܽ���ѧϰ��ѧ�Ļ���������������ijͬѧ�����IJ��ֻ�ѧ֪ʶ������Ϊ����ȷ��ѡ���ǣ� ��
A.���ʵ����ʾ�����; | B.��ѧ����Դ |
��ʯī���е����ԡ�������Ϊ�缫 ��CO���п�ȼ�ԡ������������� | ��ʯ�����ƿɵõ����͡����͡�����ú�͵� �ڿ�ȼ����¶ȴﵽ�Ż��һ����ȼ�� |
C.���ʵĹ��� | D.��ѧ���ŵ����� |
�ٷ��ӡ�ԭ�ӡ����Ӷ�����ֱ�ӹ������� �ڹ���ԭ�ӵ������������ | ��O2��һ�������� ��O2-��һ�������Ӵ�������λ����� |
A. A B. B C. C D. D
D ��������A����ʯī���е����Կ������缫��CO���п�ȼ�ԣ�������ȼ�ϣ�CO���л�ԭ�ԣ�����������������B��ʯ�����ƿɵõ����͡����͡�����ú�͵ȣ���ȼ����¶ȴﵽ�Ż�㲻һ����ȼ�գ�����Ҫ�������Ӵ�������C���������ʵ����з��ӡ�ԭ�Ӻ����ӣ�����ԭ�ӵ����е��Ӵ����磬���Ӵ����磬����D��O2��ʾ����һ�������ӣ�O2-��ʾһ�������Ӵ�������λ����ɣ���ȷ����ѡD�� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ2018����꼶��������Ӧ��ѵ����ѧ�Ծ� ���ͣ������
��ѧ�������������۲����ͼ��ʾ��ʵ�顣
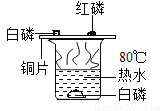
��1��ʵ���У�ͭƬ�ϵİ��ܿ�ȼ�գ��仯ѧ��Ӧ����ʽΪ ��
��2����ʵ����Ҫ������ͭƬ���õ� �ԡ�ȼ�ŵİ���Ϩ���ȥ��ͭƬ�ϵİ�ɫ���壬�ɿ���ͭƬ�����ڣ��ú�ɫ������ ���ѧʽ����
���ձ��е�ˮ��ʵ����û���������� ������ţ���
A������Ӧ�� B���������� C�������¶�
��1��4P + 5O2=2P2O5����2�����ȣ�CuO�� ��3��A ����������������Ϣ֪���v1��ʵ���У�ͭƬ�ϵİ��ܿ�ȼ�գ��䷴Ӧ�Ļ�ѧ����ʽΪ��4P+5O2 2P2O5 �� ��2����ʵ����Ҫ������ͭƬ���õĵ����ԣ�ȼ�ŵİ���Ϩ���ȥ��ͭƬ�ϵİ�ɫ���壬�ɿ���ͭƬ�����ڣ��ú�ɫ������CuO����3���ձ��е�ˮ��ʵ����û����������A.����Ӧ� �㾦�ñ�����Ҫ����ȼ��������̽����...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ2018����꼶��ѧ�ڵ�һ��������ѧ�Ծ� ���ͣ������
��ͼ��ʾ��ijͬѧ�ⶨ����������������װ�á���������װ����ϸͭ˿��
����֪��ͭ +���� 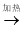 ����ͭ������ͭ�ǹ��壩
����ͭ������ͭ�ǹ��壩
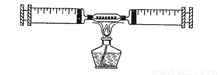
��1��������ǰ����ע������ɵ��ܱ�ϵͳ����30mL������ֹͣ������ȴ�����º��������ܱ�������ʣ�����������Ϊ_________mL���ɴ�֤������������Լռ�����������______________��
(2)��ͬ��ͬѧ��������ʵ��ʱ������ϴ�ͬѧ���ʣ������26mL������Ϊ���ܵ�ԭ������Щ����___________________________________________��
��___________________________________________________��
��3����ʵ���ܷ�ͭ˿����ľ̿��__________________��Ϊʲô�� ________________��
24 1/5 ��������ϸͭ˿�������� װ��©����δ��ȴ������ ���� ľ̿ȼ����Ȼ���������������ɶ�����̼���壬����ȷ������������������ ����������1��30mL���������������ԼΪ��30mL��1/5=6mL���������ܱ�������ʣ�����������Ϊ��30mL-6mL=24mL���ɴ�֤������������Լռ�����������1/5�� ��2�����װ��©����ϸͭ˿�������㣬û����ȴ�����¾۲죬���ܹ�������...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2018����꼶�п�ģ�⻯ѧ�Ծ� ���ͣ���ѡ��
��ͼΪ���������ʵ��ܽ�����ߡ�����˵����ȷ���ǣ�( ��

A. ���ܽ�ȴ����ҵ��ܽ��
B. t2��ʱ���ס��ҵı�����Һ�����ʵ��������
C. t1��ʱ����30g�����ʷ���50gˮ�У��õ���Һ������Ϊ80g
D. t1��ʱ���ס��ҵı�����Һ�ֱ��������100gˮ������������������
D ��������A��û��ָ���¶ȣ����Ƚ��������ʵ��ܽ�ȣ�����B��û��ָ���ı�����Һ���������Ƚϼס��ҵı�����Һ�����ʵ�����������C��t1��ʱ�����ܽ��Ϊ30g����t1��ʱ��100g��ˮ������ܽ�30g�ļף���30g����50gˮ�У��õ���Һ������=15g+50g=65g������80g����ѡ�����D��t1��ʱ���ס����ܽ����ȣ��ס��ұ�����Һ�ֱ��������100gˮ�����������������ȣ�...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2018����꼶�п�ģ�⻯ѧ�Ծ� ���ͣ���ѡ��
�̡�������ӡ�C60֮��ѧ���ַ�������һ�֡�������ӡ�N60��һ��������N60�л��۵ľ���������һ˲���ͷų�������δ���Ļ��ȼ�ϣ�N60����
A���߷��ӻ����� B���ǽ������� C���������� D�������
B ��������N60���ɷǽ���Ԫ�ص�Ԫ����ɵĴ�������ڷǽ������ʣ���ѡB���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ÷����2018����꼶��ѧ�ڵ�һ���ʼ컯ѧ�Ծ� ���ͣ������
��һ�ձ���ʢ��22.3g Na2CO3��NaCl��ɵĹ������������ˮ�ܽ⣬�Ƴ���Һ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ��
��1�����μ���73gϡ����ʱ���ų������������Ϊ g��
��2�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��������ǣ�д��ѧʽ�� ��
��3�����μ���73gϡ����ʱ����A��ʱ�����ձ���Ϊ��������Һ����ͨ��������������������ʵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ÷����2018����꼶��ѧ�ڵ�һ���ʼ컯ѧ�Ծ� ���ͣ������
��ͼ��һ�տ�����ѩ�����ϣ���ش�
��1�����ϳɷ��������л������(�����) ��
��2���ӹ���ð�����������ݣ�������˵�� ��
��1���ڢܣ�2����ѹǿ�ļ�С��CO2(������)���ܽ�ȼ�С �������� �����������1���л������������������ʻ������л������ﶼ����̼Ԫ�ء�������̼��������������ص㣬��ˣ����������������ˮ��H2O������̼Ԫ�أ�Ҳ�����л�����ǣ�C12H22O11����֬�������Ļ�ѧԪ����Ҫ��C��H��O�������л���ʴ�Ϊ���ڢ� ��2��������ܽ����ѹǿ�ļ�С����С�������ڣ�ѹǿ��С��...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ÷����2018����꼶��ѧ�ڵ�һ���ʼ컯ѧ�Ծ� ���ͣ���ѡ��
�ճ������е����б仯������һ���������������ͬ���ǣ� ��
A. ��ѩ���� B. �̻���ը C. ��ʳù�� D. �������
A �������������������ɵı仯�ǻ�ѧ�仯�������������ɵı仯�������仯��A����ѩ���ڣ������������ɣ����������仯��B. �̻���ը�������������ɣ����ڻ�ѧ�仯��C. ��ʳù�䣬�����������ɣ����ڻ�ѧ�仯��D. ������ƣ������������ɣ����ڻ�ѧ�仯����ѡA���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ĵ�ʡ����������2018����꼶��ѧ�ڵ�һ�Σ����У����Ի�ѧ�Ծ� ���ͣ���ѡ��
Ҫ�������д���IJ���������Ҫ�ٲ�����������( )
A. 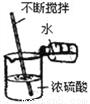 B.
B. 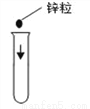 C.
C. 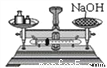 D.
D. 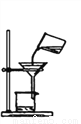
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com