
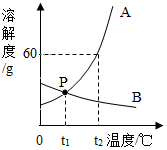
 �����ܽ�����ܽ�����ش��������⣮
�����ܽ�����ܽ�����ش��������⣮���� ���ݹ�����ܽ�����߿��ԣ��ٲ��ij������һ���¶��µ��ܽ�ȣ��Ӷ�ȷ�����ʵ��ܽ��ԣ��ڱȽϲ�ͬ������ͬһ�¶��µ��ܽ�ȴ�С���Ӷ��жϱ�����Һ�����ʵ����������Ĵ�С�����ж����ʵ��ܽ�����¶ȱ仯�ı仯������Ӷ��ж�ͨ�����½ᾧ���������ᾧ�ķ����ﵽ�ᴿ���ʵ�Ŀ�ģ�
��� �⣺��1��ͨ�������ܽ�����߿�֪��ͼ��P��ĺ����ǣ�t1�棬A��B���ʵ��ܽ����ͬ��
��2����t2��ʱ��A���ʵ��ܽ����60g������40gA���ʼ���50gˮ�ɵõ���Һ����80g��
��3��A���ʵ��ܽ�����¶ȵ����߶�����B���ʵ��ܽ�����¶ȵ����߶���С������A��B�������ʷֱ��γɵı�����Һ���¶ȶ���t1�����µ�t2��ʱ����Ȼ�DZ�����Һ����B����Һ���������������A��
��4��B���ʵ��ܽ�����¶ȵĽ��Ͷ��������������B�ı�����Һ�����ʵ������������ɲ�ȡ�Ĵ�ʩ�ǽ��£������ʣ�
�ʴ�Ϊ����1��t1�棬A��B���ʵ��ܽ����ͬ��
��2��80��
��3��B��A��
��4�����£������ʣ�
���� �����ѶȲ��Ǻܴ���Ҫ�����˹�����ܽ����������ʾ�����壬�����ݹ�����ܽ�������������ص����⣬�Ӷ������������⡢��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ����ȫ���м�����������ʡ������2017����꼶�п�ģ�⻯ѧ�Ծ� ���ͣ�ѡ�������
�ý��������ʽ����й���ʵ������ȷ���ǣ� ��
A. ��Ϊ����ͭ���ã�������ʴ������ͭ�������������ױ��浽��
B. ��Ϊ����Ʒ���γ����ܵı���Ĥ�����Բ�Ҫĥ�����ı���
C. ��Ϊ����������е�H2O��O2��Ӧ�����Գ����������渲��������������
D. ��Ϊ����п���ã����Գ���п�������ı��棬�Ա��������ܸ�ʴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������𣬲�Ҫ���ڴ������Ŵ� | |
| B�� | ȼ�ű���ʱ��ӦԶ����Ⱥ�Ϳ�ȼ�� | |
| C�� | ��Ȼ��й©��Ӧ�����رշ��Ų������̻� | |
| D�� | ���ַ�����������Ũʱ��Ӧ��ʪë����ס�ڱǣ�Ѹ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
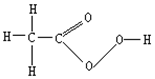 ���������ϸ���Ͳ������и�Ч������ɱ�����ã��ڿ������ǵ��ͷ��ס���ս���б��㷺Ӧ���ڻ�������������ͼΪ�� ������Ľṹʽ�������йع�������������в���ȷ���ǣ�������
���������ϸ���Ͳ������и�Ч������ɱ�����ã��ڿ������ǵ��ͷ��ס���ս���б��㷺Ӧ���ڻ�������������ͼΪ�� ������Ľṹʽ�������йع�������������в���ȷ���ǣ�������| A�� | ����������ӵĻ�ѧʽΪC2H4O3 | B�� | �����������Է�������Ϊ76 | ||
| C�� | ������������Ԫ�ص������������ | D�� | ��ȫȼ������H2O��CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʵ������ȡ����ʱ����װ������أ��ټ��װ�õ������� | |
| B�� | ����Ͳ���㵹Һ��ʱ����Һ��ӽ��̶�ʱ�����õι�����Ͳ�ڵμ�Һ�� | |
| C�� | ʵ������CO��CuO��Ӧ��ȡͭʱ����ͨCO���ٵ�ȼ�ƾ��Ƽ��� | |
| D�� | ϡ��Ũ����ʱ�������ձ��ڵ���ˮ���ٻ���ע��Ũ���ᣬ�����Ͻ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������� | B�� | ʳ��ù�� | C�� | ƻ��ե֭ | D�� | ľ��ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | N2����ʾһ����������2����ԭ�ӹ��� | |
| B�� | 2OH-����ʾ2������������ | |
| C�� | S2-����ʾ�����Ӵ�2����λ����� | |
| D�� | H2S����ʾ1�������������2����Ԫ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com