�Ķ����в��ϣ��������̽����
����Լ��92%��CaCO
3��4%�ĵ����ʣ������ᣩ�Լ�ˮ���������ʣ�������ĥ�ɵ�����۾��а������ݵ�ҽ�ü�ֵ�����ӡ�ÿ���������桷����¶�ֽ��г��ϴ��ڴ������Ƿ�ð�������ۣ�������������Һȥ�����DZ�����л����˫��ˮȥ������ĺ�ɫ�صȣ�Ȼ��ĥ�ɷۼ��ɣ���ij�о���ѧϰС��Ա��ص�һ����������۽���������̽����
̽��һ��������۵���Ҫ�ɷ֣���ܰ��ʾ������������Ũ���Ტ���Ȼ���ֻ�ɫ���ɫ ��
| ��� |
ʵ������ |
ʵ������ |
ʵ����� |
| �� |
ȡ������Ʒ���ձ��У��μ�ϡ���� |
����������� |
������ |
| �� |
�ò�˿պȡ������Һ���ھƾ��������� |
����Ϊש��ɫ |
��Һ�к��и����� |
| �� |
��ȡ������Ʒ���ձ��У���ˮ����ܽ⣬�μ� ��ˮ���� ��ˮ���� |
����ɫ���� |
��Ʒ�в����е��� |
| �� |
��ȡ������Ʒ���ձ��У���ˮ�ܽ⣬���á����ˣ�����Һ�м�������Ũ���ᣬ�������� |
������ɫ���� ������ɫ���� |
��Ʒ�к�����
�������ᣩ |
��1��С����Ϊ����ʵ��١��ڿ����ƶ���Ʒ��һ������̼��ƣ�С�Dz�ͬ����۵㣮Ϊ��ȷ����Ʒ�к���̼��ƣ�����Ҫ������ɵ�ʵ���ǣ�д��ʵ�鷽��������
�������ɵ�����ͨ��ʢ�г���ʯ��ˮ���Թ��У��������֤���Ǻ���̼���
�������ɵ�����ͨ��ʢ�г���ʯ��ˮ���Թ��У��������֤���Ǻ���̼���
��
��2����������ʵ�飬�����жϸ������Ϊ
��
��
����桱�١�����
̽���������������̼��ƺ����IJⶨ
������1��С������ͼ1��ʾװ�òⶨ���������̼��Ƶĺ�����ʵ�鷽�����£�
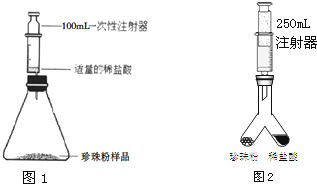
��1�����װ�������ԣ���ͼ����װ���ú�ע���������������ǻ�����Ħ�������أ���ͬ���ӿ̶�1mL�����������̶�20mL����
�ɿ�ע�����������������»ص�1�̶ȴ�
�ɿ�ע�����������������»ص�1�̶ȴ�
������������������������ã�
��2������ƿ��װ��0.4g�����������Ʒ��ע����������12mL��ϡ���ᣬ�ٴ�����װ�ã�
��3����������ƿ��ע������ϡ���ᣬ���ζ���ƿ����û�����ݲ�����¼ע�����̶�Ϊ92mL��
��4�����ݴ�������ʵ������в���CO
2�����Ϊ
80
80
mL���ٸ���CO
2���ܶȣ�Լ2g/L��������������������̼��Ƶ��������Ӷ�������������̼��Ƶĺ�����
������2��ͬ���С��ͬѧ���������һ��װ�ã���ͼ2��ʵ�鷽�����£�
��1�����װ�������ԣ�
��2������ͼY��װ�������װ��1.1g�����������Ʒ���ҹ��ڵ���һ������ϡ���ᣬ��Ͳ�����Ϊ250mL�������Ƶ��ײ����ٴ�����װ�ã�
��3���跨ʹ���������ڵ�ҩƷ��ϣ���Ӧ�������ȡ��Ͳ����Ϊ220mL��
��4�����ݴ������������������̼��Ƶ�������������д��������̣����ս������С�����1λ��
����ע��������Ϊ220mL�������ɵĶ�����̼������=0.22L��2g/L=0.44g
���������̼��Ƶ�����Ϊx
CaCO
3+2HCl=CaCl
2+H
2O+CO
2��
10044
x 0.44g
=
x=1g
����������̼��Ƶ���������
��100%��90.9%
���������̼��Ƶ�����������90.9%
����ע��������Ϊ220mL�������ɵĶ�����̼������=0.22L��2g/L=0.44g
���������̼��Ƶ�����Ϊx
CaCO
3+2HCl=CaCl
2+H
2O+CO
2��
10044
x 0.44g
=
x=1g
����������̼��Ƶ���������
��100%��90.9%
���������̼��Ƶ�����������90.9%
��ʵ�鷴˼��
��1��������Ͳ�����أ���������ʵ�鷽��������۵��������ٱ�����Ҫ����Ϊʲô��
������������̫�����ɵ����峬����Ͳ���������ƣ����������ɶ�����̼�����
������������̫�����ɵ����峬����Ͳ���������ƣ����������ɶ�����̼�����
��
��2��������2���еڣ�3�������ʵ������ҩƷ�Ļ�ϣ�
��Y��װ��������б��ֱ��ʹϡ����������߹���������۳�ֽӴ�
��Y��װ��������б��ֱ��ʹϡ����������߹���������۳�ֽӴ�
��
��3��С����Ƶ�װ����С���ȣ��к��ŵ㣿
����Ҫ���Ǽ��������Բ������������Ӱ�죨����������ۻ��ʱ������ֳ��������1��������ʱ���ܻ��˳��ֳ�������
����Ҫ���Ǽ��������Բ������������Ӱ�죨����������ۻ��ʱ������ֳ��������1��������ʱ���ܻ��˳��ֳ�������
��д��1�㼴�ɣ���




 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
 3X+4H2O����X�Ļ�ѧʽΪ
3X+4H2O����X�Ļ�ѧʽΪ