
| �ɷ� | ������ | ��֬ | ���� | ˮ��ά����B��� |
| �������� | Լ10% | Լ5% | Լ80% | Լ5% |
 2CO2+3H2O��
2CO2+3H2O�� 2CO2+3H2O��
2CO2+3H2O��

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2007��ȫ���п���ѧ���⣺��8��Ԫ �����ͽ������ϣ������棩 ���ͣ������
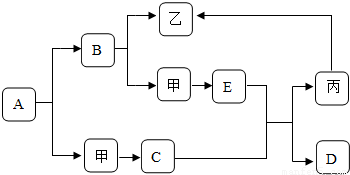 ��2009?�Ű�����ͼ�dz��л�ѧ�г������ʼ��ת����ϵ�����мס��ҡ���Ϊ���ʣ�A��B��C��D��EΪ�������A��B�����Ԫ����ͬ��D��E�����Ԫ��Ҳ��ͬ����֪C������Ϊ��ɫ��ĩ��C��E�ڸ��������¿����ɱ���D��D��ʹ�����ʯ��ˮ����ǣ����෴Ӧ���������ַ�Ӧ��������������ȥ�����ƶϣ�
��2009?�Ű�����ͼ�dz��л�ѧ�г������ʼ��ת����ϵ�����мס��ҡ���Ϊ���ʣ�A��B��C��D��EΪ�������A��B�����Ԫ����ͬ��D��E�����Ԫ��Ҳ��ͬ����֪C������Ϊ��ɫ��ĩ��C��E�ڸ��������¿����ɱ���D��D��ʹ�����ʯ��ˮ����ǣ����෴Ӧ���������ַ�Ӧ��������������ȥ�����ƶϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���з�ɽ���п���ѧһģ�Ծ��������棩 ���ͣ������
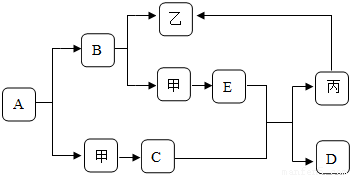 ��2009?�Ű�����ͼ�dz��л�ѧ�г������ʼ��ת����ϵ�����мס��ҡ���Ϊ���ʣ�A��B��C��D��EΪ�������A��B�����Ԫ����ͬ��D��E�����Ԫ��Ҳ��ͬ����֪C������Ϊ��ɫ��ĩ��C��E�ڸ��������¿����ɱ���D��D��ʹ�����ʯ��ˮ����ǣ����෴Ӧ���������ַ�Ӧ��������������ȥ�����ƶϣ�
��2009?�Ű�����ͼ�dz��л�ѧ�г������ʼ��ת����ϵ�����мס��ҡ���Ϊ���ʣ�A��B��C��D��EΪ�������A��B�����Ԫ����ͬ��D��E�����Ԫ��Ҳ��ͬ����֪C������Ϊ��ɫ��ĩ��C��E�ڸ��������¿����ɱ���D��D��ʹ�����ʯ��ˮ����ǣ����෴Ӧ���������ַ�Ӧ��������������ȥ�����ƶϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�Ƹ����ˮ�ذͺ���ѧ�п���ѧģ���Ծ��������棩 ���ͣ������
 ��2009?�Ű�����ͼ�dz��л�ѧ�г������ʼ��ת����ϵ�����мס��ҡ���Ϊ���ʣ�A��B��C��D��EΪ�������A��B�����Ԫ����ͬ��D��E�����Ԫ��Ҳ��ͬ����֪C������Ϊ��ɫ��ĩ��C��E�ڸ��������¿����ɱ���D��D��ʹ�����ʯ��ˮ����ǣ����෴Ӧ���������ַ�Ӧ��������������ȥ�����ƶϣ�
��2009?�Ű�����ͼ�dz��л�ѧ�г������ʼ��ת����ϵ�����мס��ҡ���Ϊ���ʣ�A��B��C��D��EΪ�������A��B�����Ԫ����ͬ��D��E�����Ԫ��Ҳ��ͬ����֪C������Ϊ��ɫ��ĩ��C��E�ڸ��������¿����ɱ���D��D��ʹ�����ʯ��ˮ����ǣ����෴Ӧ���������ַ�Ӧ��������������ȥ�����ƶϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɽ��ʡ�������п���ѧģ���Ծ��������棩 ���ͣ������
 ��2009?�Ű�����ͼ�dz��л�ѧ�г������ʼ��ת����ϵ�����мס��ҡ���Ϊ���ʣ�A��B��C��D��EΪ�������A��B�����Ԫ����ͬ��D��E�����Ԫ��Ҳ��ͬ����֪C������Ϊ��ɫ��ĩ��C��E�ڸ��������¿����ɱ���D��D��ʹ�����ʯ��ˮ����ǣ����෴Ӧ���������ַ�Ӧ��������������ȥ�����ƶϣ�
��2009?�Ű�����ͼ�dz��л�ѧ�г������ʼ��ת����ϵ�����мס��ҡ���Ϊ���ʣ�A��B��C��D��EΪ�������A��B�����Ԫ����ͬ��D��E�����Ԫ��Ҳ��ͬ����֪C������Ϊ��ɫ��ĩ��C��E�ڸ��������¿����ɱ���D��D��ʹ�����ʯ��ˮ����ǣ����෴Ӧ���������ַ�Ӧ��������������ȥ�����ƶϣ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com