
����ʵ�鷽����Ʋ���������( )
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ��ȥ������̼�е�һ����̼ | ������ͨ������������Һ |
B | ����������Ƿ�����Ԫ�� | ��ȼ���ڻ����Ϸ���һ������ձ����۲����� |
C | �������ߺ���ë�� | �ֱ����գ�����ζ |
D | ��ȥʳ���е���ɳ | �ܽ⡢���ˡ����� |
A. A B. B C. C D. D
A ��������A�����ӵ�ԭ��ֻ���ӣ������ӡ��������Լ�ֻ�������ʷ�Ӧ����������Ҫ����Ҫ�ɷַ�Ӧ��ͬʱ���������µ����ʡ�������̼�����������Ʒ�Ӧ�����ܴﵽ����Ŀ�ģ��ʴ��� B�������غ㶨�ɿ�֪��Ӧǰ��Ԫ������䣻�������ȼ���ڻ����Ϸ���һ������ձ����ձ��ڱ���ˮ���ɣ�˵����ȼ��������Ԫ�أ�����ȷ�� C����ȼ����ë�ܷ����ս���ë��ζ��������û�У�����ȷ�� D�������ܽ⡢��...
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡƽ��ɽ���е��в��ԣ�һ����ѧ�Ծ� ���ͣ���ѡ��
���෨�ǻ�ѧѧϰ���о�����Ҫ����֮һ�����з����ȷ���ǣ� ��
A. �Σ����С�մ������� B. �Ͻ���������ͭ��Ӳ��
C. ������Ȫˮ��ʯ�͡����� D. �ǽ���Ԫ�أ���(Hg)����(F)����(Si)
D ��������A�����ǵ�����������Ӻ�������ӵĻ�������С�մ��������������Σ���ȷ��B���Ͻ����ڽ����м����ۺ�ijЩ������ǽ����γɵ����ʡ���������ͭ��Ӳ�����ǺϽ���ȷ�� C�����������������������ɡ���Ȫˮ��ʯ�͡��������ǻ�����ȷ��D������Ԫ��һ�㶼���С��ġ����ԣ������⣩������ѡD���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2018����꼶�п�ģ�⻯ѧ�Ծ� ���ͣ���ѡ��
�̲��ǰ��յ��ز�������ܶ࣬�̲��ǽ����ʵIJ�Ҷ�������ƻ����н����پ����ࡢ�決���ɡ���Ҷ�Ļ�ѧ�ɷ���Ҫ�Dz��(C8 H10 N4O2)���������ἰ�����͵ȣ�����˵��������ǣ� ��
A. ���������Ԫ����� B. һ���������к�24��ԭ��
C. ��Ҷ�ǻ���� D. �������Ԫ�ص������������
D ��������A. �����C��H��N��Y����Ԫ����ɣ���ȷ��B. һ���������к�8+10+4+2=24��ԭ�ӣ���ȷ��C. ��Ҷ����Ҫ�ɷ��Dz����������������ʣ��ǻ�����ȷ�� D. �����̼Ԫ�ص��������������ѡD���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�����е���У2018����꼶��ѧ�ڵڶ���������ѧ�Ծ� ���ͣ�������
��ѧʩ����ʵ��ũҵ��������Ҫ�ֶΡ�����泥���NH4NO3����һ�ֳ��õĵ��ʣ����е�Ԫ�ء���Ԫ�ء���Ԫ�ص�������Ϊ_____________����Ҫ����200g������������Ϊ5%���������Һ����Ҫ����淋�����Ϊ_________g,��Ҫˮ������Ϊ_______g.
7�s1�s12 10 190 ��������(1).��������Ԫ�ص�������=�����ԭ��������ԭ�Ӹ�����֮�ȣ���NH4NO3���е�Ԫ�ء���Ԫ�ء���Ԫ�ص�������Ϊ14��2:4:16��3= 7�s1�s12�����ʵ�����=��Һ�����������ʵ�������������Ҫ����200g������������Ϊ5%���������Һ����Ҫ����淋�����Ϊ�� 200g��5%=10g����Һ�����ʺ��ܼ���ɣ�����ˮ��������=200g-10g=190g. ...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�����е���У2018����꼶��ѧ�ڵڶ���������ѧ�Ծ� ���ͣ��ۺ���
�û�ѧ�����ʾ��
(1)�����к�����������_________________(2)3��笠�����__________��
(3)����ʯ��ˮ�е�����_________________(4)2����������_________________.
(5)�����������γɵı���Ĥ_________________.(6)ʯ��ʯ����Ҫ�ɷ�_________________.
(7)+4����Ԫ�ص�������Ļ�ѧʽ__________��
N2 3NH4+ Ca(OH)2 2Fe2+ Al2O3 CaCO3 SO2 ����������1�������к������������ǵ���N2����2��3��笠����ӿ��Ա�ʾΪ3NH4+�� (3)����ʯ��ˮ�е�������������Ca(OH)2����4��2���������ӿ��Ա�ʾΪ2Fe2+��(5)�����������γɵı���Ĥ������Al2O3��(6)ʯ��ʯ����Ҫ�ɷ���̼���CaCO3����7��+6����Ԫ�غ�-2�۵���Ԫ���γɵĻ�����...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�����е���У2018����꼶��ѧ�ڵڶ���������ѧ�Ծ� ���ͣ���ѡ��
����β�����е��������һ����̼���ж����壬�ڴ����IJ����¶�β���������۹�����ͼ ����˵������ȷ����
����˵������ȷ����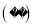
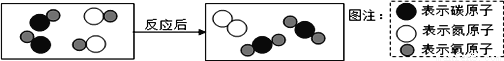
A. ͼ�е��ʵĻ�ѧʽΪ
B. �÷�Ӧʹ�к�����ת��Ϊ������
C. ���ɵ��ʺͻ������������Ϊ7��11
D. �����ڷ�Ӧǰ��ѧ���ʲ���
C ��������A��ͼ��ֻ��һ�ֵ��ʣ�Ϊ����Nԭ�ӹ��ɵĵ��ʷ��ӣ����Ըõ��ʵĻ�ѧʽΪN2����ȷ��B���÷�Ӧ�ķ�Ӧ��ֱ�Ϊ��CO���ӹ����ж���CO���壬NO���ӹ����ж���NO���壻��Ӧ��������Ϊ��CO2���ӹ�������CO2���壬N2���ӹ�������N2���壬��ȷ��C����ͼʾ��֪���÷�Ӧ�ķ���ʽΪ��2CO+2NO2CO2+N2�����ɵ��ʺͻ������������Ϊ��28��88��=7��22������D�������Ļ�...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������2018�������ѧ�п���ϰ ˮ����� ר����ϰ�� ���ͣ������
���ݵ��ˮ��ʵ��װ��ͼ����������⣺
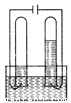
(1)ָ����Դ���������������Ϊ____���ұ�Ϊ____��������ˮ������������ƣ�Ŀ����Ϊ��____��
(2)����Թ��в�������___�����������ķ�����____���ұ��Թ��в�������____�����������ķ�����____��������ʵ����ʵ�ó����ۣ�˵��ˮ����______��ɵġ�
���� ���� ��ǿˮ�ĵ����� ���� ��ȼ�ŵ�ľ�������ܿ� ���� �Ѵ����ǵ�ľ��������� �⡢������Ԫ�� ����������1����ͼ��֪����ߵ��Թ��в����������������ұ��Թܲ������������2����������Թܲ��������������ұ��Թܲ������������������Ϊ�������ұ�Ϊ������ˮ�ĵ����Բ��ã��ʼ���������������Һ�ĵ����ԣ� ��2������Թܲ��������������������������ķ����ǰ�ȼ�ŵ�ľ�������ܿڣ�����������ı�...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�人��2017-2018ѧ����꼶��ѧ��12�¿���ѧ�Ծ� ���ͣ�������
С��Ϊ�˲ⶨ��������̼��Ƶĺ�������������11.0g��������װ��100.0g����ϡ������ձ��У���ַ�Ӧ�����ձ�������������Ϊ106.6g(���ʲ��μӷ�Ӧ��Ҳ���������ʺ���)
����㣺
(1)����������̼������______
(2)�ü�������Ʒ��̼��Ƶ���������(��ȷ��0.1%)
(1)4.4g (2)90.9% �����������⿼���˸��ݻ�ѧ��Ӧ����ʽ�ļ��㡣 ���������غ㶨�ɣ�������̼������Ϊ��11.0g+100.0g-106.6g=4.4g �裺ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx�� CaCO3+2HCl�TCaCl2+H2O+CO2�� 100 44 x 4.4g x=10g ̼��Ƶ���������=��100%= 90.9%�� �𣺸�...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�人��2017-2018ѧ��Ԫ��ģ�⻯ѧ�Ծ� ���ͣ��ƶ���
A~G�dz�����ѧ�ij������ʣ���ת����ϵ��ͼ��ʾ(���ַ�Ӧ��������Ӧ��������ȥ)��A��B������Ϊ��ɫ���������Ԫ����ͬ��C�Ǵ���ʯ����Ҫ�ɷ֣�D��E��Ϊ��ɫ���塣��ش��������⣺
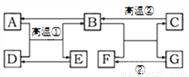
(1)F�Ļ�ѧʽ��____________��
(2)��Ӧ�۵Ļ�����Ӧ������____________��
(3)д����Ӧ�ٵĻ�ѧ����ʽ_____________________��
(4)д��A��һ����;��____________��
Ca(OH)2 ���Ϸ�Ӧ Fe3O4+4CO3Fe+4CO2 ȼ�ϣ���ұ�������� ������������Ϊ��ͼʽ�ƶ��⣬��ɴ��⣬�ؼ��Ǹ������������������ͻ�ƿڣ�ֱ�ӵó��й����ʵĻ�ѧʽ��Ȼ��������������Ͽ�ͼ���ƶϵó��������ʵĻ�ѧʽ������Ľ���ͻ�ƿ���C�Ǵ���ʯ����Ҫ�ɷ֡� C�Ǵ���ʯ����Ҫ�ɷ֣�C��̼��ƣ�C�ڸ�����������B��A��B������Ϊ��ɫ���������Ԫ����ͬ����A��B����...�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com