
| ʵ����� | �¶� | ��ȴ�����������������������ˣ� |
| 1 | T1 | 48.5 |
| 2 | T2 | 44.9 |
| 3 | T3 | 43.1 |
| 4 | T4 | 43.1 |
���� ������Ŀ���������ݷ����ͽ��ע��Ҫ�۳�������������
��� �⣺��1����һ�μ��Ⱥ�������ʣ������������=55.7g-32.5g=23.2g��ʧȥ�Ľᾧˮ������=55.7g-48.5g=7.2g��
��2���ڶ��μ��Ⱥ������������Ľᾧˮ=44.9g-43.1g=1.8g��������������Ϊ44.9g-32.5g=12.4g����ᾧˮ����������Ϊ$\frac{1.8g}{12.4g}$��100%��14.5%��
��3��ԭ��Ʒ�Ļ�ѧʽΪNa2CO3•nH 2O��
ˮ������Ϊ55.7g-43.1g=12.6g��
̼���Ƶ�����Ϊ43.1g-32.5g=10.6g
��106����18n��=10.6g��12.6g��n=7
����ԭ��Ʒ�Ļ�ѧʽΪNa2CO3•7H 2O��
�ʴ�Ϊ����1��23.2g��7.2g��
��2��14.5%
��3��Na2CO3•7H 2O��
���� �������ݷ���ʱ������Ҫ��ȷ���ݱ������������壬����Ҫ�۳��������ݣ�


 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
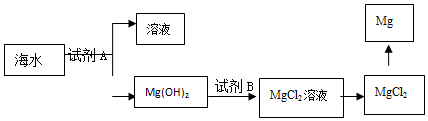
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��չ�� | B�� | ������ | ||
| C�� | Ӳ�ȴ� | D�� | ��Ư���Ľ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 30% | B�� | 40% | C�� | 50% | D�� | 60% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ϡ���� | B�� | ϡ���� | C�� | ��������Һ | D�� | ʯ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �����Ͷ�����̼ | B�� | ��ۺͿ��� | C�� | ��Ȼ���Ϳ��� | D�� | һ����̼������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | īˮ | C�� | ���� | D�� | ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com