
| ||
| ||


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
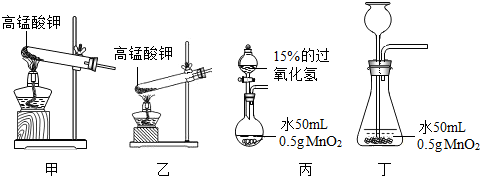
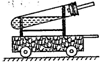 20������һ��ij�ο���������һ����Ȥ�ı�������˭�����ֳ�һ�����еľ�����Զ����������ѧ��ȤС����������ͼ��ʾ��һ��С�����������Թ��м��������ּ����������ӣ�ͨ���������������ʱ�ķ�������ʹС����ǰ�˶��ˣ�����ѡ����Լ�����п�ۣ���ͭ�ۣ������ۣ���10%��ϡ���ᣬ��10%��ϡ���ᣩ
20������һ��ij�ο���������һ����Ȥ�ı�������˭�����ֳ�һ�����еľ�����Զ����������ѧ��ȤС����������ͼ��ʾ��һ��С�����������Թ��м��������ּ����������ӣ�ͨ���������������ʱ�ķ�������ʹС����ǰ�˶��ˣ�����ѡ����Լ�����п�ۣ���ͭ�ۣ������ۣ���10%��ϡ���ᣬ��10%��ϡ���ᣩ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
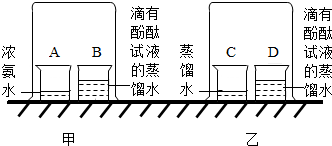
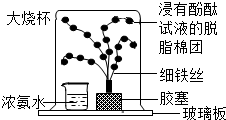
 15��ij��ѧ������ȤС�飬��չ������������
15��ij��ѧ������ȤС�飬��չ������������ ʵ���������Һ��pH�仯��������ͼ��ʾ��
ʵ���������Һ��pH�仯��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com